I. Introduction
In our daily lives, sodium bicarbonate, is a familiar and versatile compound. One of the most common sources of sodium bicarbonate in daily life is baking soda, which is readily available in grocery stores.

We encounter it in various forms and applications, from baking fluffy cakes and crispy cookies in the kitchen to soothing heartburn as an over-the-counter remedy.
It even plays a role in household cleaning, helping to scrub away grime and odors.
One of the most fundamental properties of sodium bicarbonate that underpins many of its uses is its solubility in water.
Have you ever wondered why this seemingly ordinary white powder readily disappears when stirred into water?
What forces and molecular interactions are at play that allow sodium bicarbonate to dissolve so easily?
In this exploration, we will delve deep into the world of chemistry to uncover the reasons behind the solubility of sodium bicarbonate in water, shedding light on the fascinating processes that occur at the molecular level.
Understanding this not only satisfies our scientific curiosity but also provides valuable insights into how we can make the most of this remarkable substance in countless practical applications.
II. The Basics of Sodium Bicarbonate
Sodium bicarbonate, with the chemical formula NaHCO3, is a white crystalline powder that is odorless and has a slightly alkaline taste.
It is an acid salt, formed by the partial neutralization of carbonic acid (H2CO3) with sodium hydroxide (NaOH). For the details of its producing method, please read article: what is sodium bicarbonat.
Structurally, it consists of a sodium cation (Na⁺) and a bicarbonate anion (HCO₃⁻). The bicarbonate anion is amphiprotic, meaning it can both donate and accept a proton, which gives sodium bicarbonate its unique acid-base properties. In water, it can act as a weak base, accepting a proton from water molecules to form carbonic acid, which then decomposes into water and carbon dioxide. This property is crucial in many of its applications, such as in baking, where the release of carbon dioxide gas helps dough rise and gives baked goods their characteristic texture.
III. Solubility: The Phenomenon
A. Observing Sodium Bicarbonate in Water
When a small amount of sodium bicarbonate is added to water, several immediate and observable changes take place.
At first glance, one notices that the fine white powder begins to disappear as it is stirred into the liquid. The dissolution process is relatively brisk, with the sodium bicarbonate particles seemingly being pulled apart and incorporated into the water.
As the powder dissolves, a faint fizzing or bubbling can often be detected. This is due to a chemical reaction that occurs: the bicarbonate ions react with water molecules, leading to the formation of carbonic acid (H2CO3), which is unstable and quickly decomposes into water and carbon dioxide gas (CO2). The release of these tiny gas bubbles gives the visual cue of the fizz.

The solution, which was initially clear like pure water, gradually takes on a slightly cloudy or hazy appearance. This is because the dissolved sodium bicarbonate alters the refractive index of the water, and any undissolved microparticles or the newly formed reaction products contribute to the turbidity.
Overall, the process of sodium bicarbonate dissolving in water is a dynamic one, involving both physical dissolution and chemical reactions that can be easily witnessed with the naked eye.
B. Comparison with Other Compounds
To better understand the solubility of sodium bicarbonate, it is instructive to compare it with other related compounds.
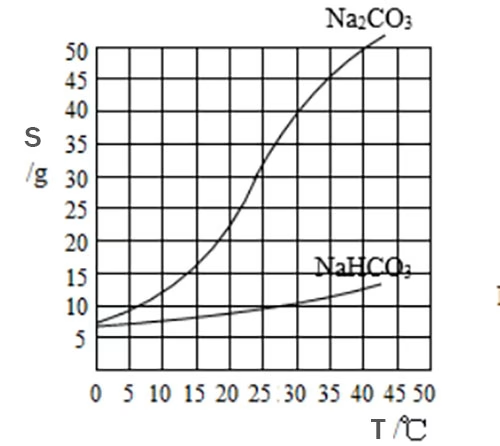
Take sodium carbonate (Na2CO3), for instance. Sodium carbonate is also highly soluble in water, but it exhibits some differences. When the same amount of sodium carbonate and sodium bicarbonate are added to separate containers of water at room temperature, sodium carbonate tends to dissolve more rapidly and completely(Please read article: Sodium Carbonate vs Sodium Bicarbonate for detail explanation on their solubility difference.)
Another compound for comparison is sodium chloride (NaCl), the common table salt. Sodium chloride is extremely soluble in water and dissolves in a rather straightforward manner. It does not produce the fizzing or bubbling seen with sodium bicarbonate as there is no acid-base reaction involved. When salt is added to water, it simply dissociates into sodium cations (Na⁺) and chloride anions (Cl⁻), and these ions disperse evenly throughout the water due to the polar nature of water molecules. The solubility of sodium chloride is much higher than that of sodium bicarbonate; at 20°C, approximately 36 grams of sodium chloride can dissolve in 100 grams of water. This vast difference in solubility and dissolution behavior highlights the unique chemical and physical properties of sodium bicarbonate that set it apart from other common sodium-containing compounds.
IV. The Science Behind Solubility
A. Molecular Structure and Polarity
The molecular structure of sodium bicarbonate plays a crucial role in its solubility in water. As mentioned earlier, it consists of a sodium cation (Na⁺) and a bicarbonate anion (HCO₃⁻). The bicarbonate anion has a relatively complex structure, with a central carbon atom bonded to three oxygen atoms and a hydrogen atom. This arrangement gives the anion a certain degree of asymmetry.
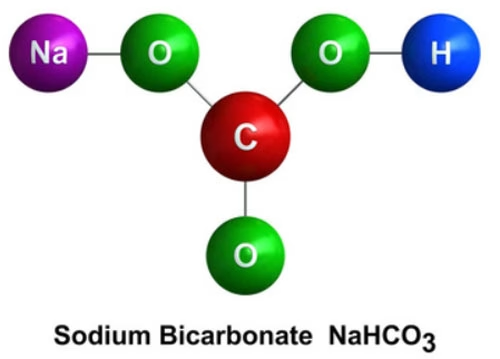
Water, on the other hand, is a polar molecule, with the oxygen atom being more electronegative than the hydrogen atoms, resulting in a partial negative charge on the oxygen and partial positive charges on the hydrogens. When sodium bicarbonate is added to water, the polar water molecules interact with the ions in the compound. The positively charged sodium ions are attracted to the negative end of the water molecules (the oxygen atoms), while the negatively charged bicarbonate ions interact with the positive ends of the water molecules (the hydrogen atoms). This electrostatic attraction between the ions and the water molecules helps to break the ionic bonds holding the sodium bicarbonate lattice together and allows the individual ions to become surrounded by water molecules, a process known as hydration. The polar nature of both the water and the ions in sodium bicarbonate thus facilitates the dissolution process.
B. Ionization in Water
Once sodium bicarbonate is added to water, it undergoes ionization. The compound is a strong electrolyte, which means it dissociates almost completely into its constituent ions in aqueous solution.
The first step of ionization is the separation of sodium bicarbonate into sodium ions (Na⁺) and bicarbonate ions (HCO₃⁻), as represented by the equation:
NaHCO₃ → Na⁺ + HCO₃⁻.
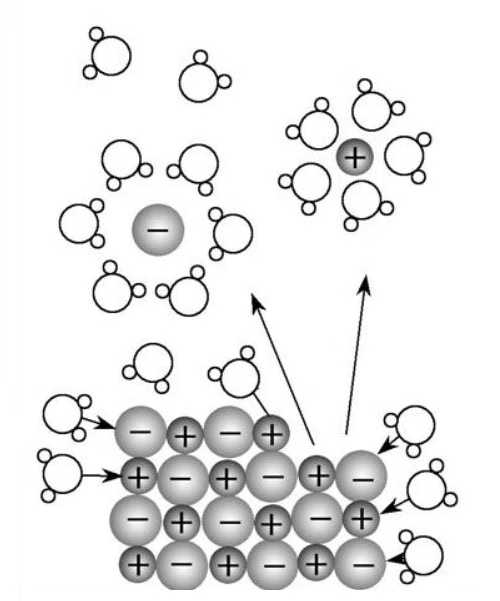
The sodium ions, being cations, are immediately hydrated by the surrounding water molecules. Each sodium ion attracts a shell of water molecules, with the oxygen atoms of the water oriented towards the positive sodium ion. This forms hydrated sodium ions, often written as [Na(H₂O)ₙ]⁺, where n represents the number of water molecules associated with the ion.
The bicarbonate ions also interact with water. Although they do not ionize completely like strong acids or bases, a small fraction of bicarbonate ions further react with water in a reversible process. Some bicarbonate ions donate a proton (H⁺) to water, forming carbonic acid (H₂CO₃) and hydroxide ions (OH⁻), while others accept a proton from water to form carbonate ions (CO₃²⁻) and hydronium ions (H₃O⁺). This complex behavior of bicarbonate ions in water is due to their amphiprotic nature and contributes to the overall chemical equilibrium in the solution.
Overall, the ionization of sodium bicarbonate and the subsequent interactions of the ions with water are essential steps in its dissolution.
C. Role of Temperature and Pressure
Temperature and pressure are two external factors that significantly influence the solubility of sodium bicarbonate in water.
As temperature increases, the solubility of sodium bicarbonate generally rises. This is because higher temperatures provide more thermal energy to the system. The added energy causes the water molecules to move more vigorously, increasing their kinetic energy. With greater molecular motion, the water molecules are better able to overcome the attractive forces between the ions in the sodium bicarbonate lattice and break them apart. Additionally, the increased temperature also affects the hydration shells around the ions. It allows for more water molecules to interact with the ions, stabilizing them in solution. For example, at 20°C, about 9.6 grams of sodium bicarbonate can dissolve in 100 grams of water, but at 60°C, the solubility increases to around 21.8 grams.

In terms of pressure, the effect on the solubility of sodium bicarbonate is relatively minor compared to temperature. However, under higher pressures, the solubility can increase slightly. According to Henry’s law, the solubility of a gas in a liquid is proportional to the partial pressure of the gas above the liquid. When pressure is increased, more gas molecules (in this case, carbon dioxide that can be released from the bicarbonate) can be forced into the solution, which in turn can affect the overall solubility equilibrium. Although the direct impact of pressure on the solubility of solid sodium bicarbonate is not as pronounced as temperature, it still plays a role in certain scenarios, such as in industrial processes where precise control of solubility is required. Understanding the combined effects of temperature and pressure provides a more comprehensive picture of how sodium bicarbonate behaves in aqueous environments under different conditions.
V. Applications Relying on Solubility
A. Medical and Health Aspects
In the medical field, the solubility of sodium bicarbonate is carefully harnessed. In intravenous (IV) solutions, precise control of its solubility is essential.

Sodium bicarbonate injections are used to treat specific types of acidosis, a condition where the body’s blood pH becomes too acidic. Pharmacists and medical professionals must accurately calculate the amount of sodium bicarbonate to dissolve in the appropriate volume of sterile water or saline solution. The solubility characteristics determine how quickly and completely the compound can be incorporated into the solution to achieve the desired therapeutic effect. When administered intravenously, the dissolved sodium bicarbonate helps to neutralize excess acid in the bloodstream, restoring the body’s pH balance.
B. Industrial and Environmental Uses
Industrially, sodium bicarbonate’s solubility is exploited in numerous processes.
In water treatment plants, it is used to adjust the pH of water. Municipal water supplies sometimes have acidic components that need to be neutralized to prevent pipe corrosion and ensure the water is safe for distribution. Sodium bicarbonate, with its solubility properties, can be added in controlled amounts to raise the pH to an acceptable level.

In industrial wastewater treatment, it plays an even more significant role. For example, in electroplating industries, the wastewater often contains heavy metal ions and is highly acidic. Sodium bicarbonate is dissolved in the wastewater, where it reacts with the acid to increase the pH. This change in pH causes the heavy metal ions to precipitate out as hydroxides, which can then be removed through filtration, effectively purifying the wastewater.
In the environmental sector, sodium bicarbonate is used in soil remediation. In areas with acidic soil, which can limit plant growth, soluble sodium bicarbonate can be applied. As it dissolves in soil moisture, it gradually neutralizes the acidity, creating a more favorable environment for plants to thrive. This simple yet effective use of its solubility helps to rejuvenate damaged or infertile soil, promoting ecological restoration.

VI. Conclusion
In conclusion, the solubility of sodium bicarbonate in water is a fascinating topic that intertwines the principles of chemistry with a multitude of practical applications.
At the molecular level, the polar nature of water and the ionic character of sodium bicarbonate, along with its amphiprotic bicarbonate anion, work in harmony to drive the dissolution process.
Temperature and pressure further modulate this solubility, providing additional control variables in various settings.

From the fluffiness of our favorite baked goods in the kitchen to life-saving medical treatments and crucial environmental remediation efforts, the solubility of sodium bicarbonate underpins its versatility.
However, as with any chemical, proper handling and safety precautions are essential to fully enjoy its benefits while minimizing potential risks.
By understanding the science behind why sodium bicarbonate dissolves in water, we are empowered to make informed decisions in our daily lives, in industries, and in scientific research, unlocking the full potential of this remarkable compound and paving the way for innovation and improved quality of life.
Expand reading:
Why is sodium bicarbonate used for baking.

