I.Introduction to Sodium Monofluorophosphate
Sodium monofluorophosphate is indeed a remarkable compound. It appears as a white solid with a high solubility in water. This property makes it highly accessible for various applications.
One of its primary uses is as an anticaries agent and tooth desensitizer. The compound plays a crucial role in dental care products. Research shows that it helps in preventing tooth decay by strengthening the enamel.
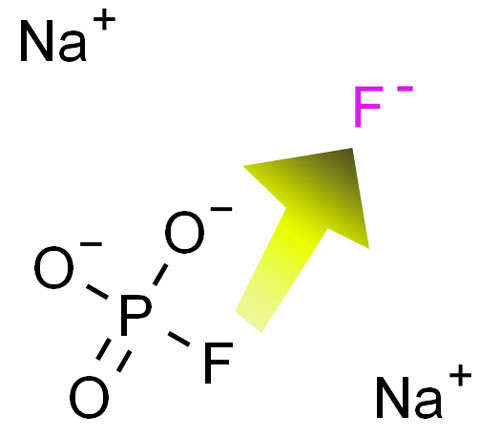
With a molecular formula of Na2PO3F and a molecular weight of 143.94, sodium monofluorophosphate has specific characteristics that contribute to its effectiveness. For instance, its solubility allows it to be easily incorporated into toothpaste formulations.
The compound is also known for its lack of corrosive properties. This is an important feature as it ensures that it does not damage the teeth or other materials it comes in contact with. Additionally, a 2% aqueous solution of sodium monofluorophosphate has a pH value ranging from 6.5 to 8.0, which is relatively neutral and safe for use.
II.The Role of Fluoride Ion
A.Impact on Chemical Properties
The fluoride ion plays a crucial role in determining the properties of sodium monofluorophosphate chemical reaction. It influences the reactivity and stability of the compound. Fluoride ion can enhance the stability of sodium monofluorophosphate by forming strong bonds. This leads to a decreased reactivity with other substances. For example, research shows that the presence of fluoride ion reduces the likelihood of sodium monofluorophosphate reacting with acidic substances. This is important in dental care products as it ensures that the compound remains stable in the presence of various oral fluids.

Moreover, the fluoride ion can also affect the chemical reactions that sodium monofluorophosphate participates in. It can act as a catalyst in certain reactions, speeding up the process. However, in other cases, it can inhibit reactions by blocking certain active sites. For instance, in the reaction with calcium ions in tooth enamel, the fluoride ion helps in the formation of a more stable compound, strengthening the enamel and preventing tooth decay.
B.Effect on Physical Characteristics
The presence of fluoride ion also has a significant impact on the physical properties of sodium monofluorophosphate. One of the most notable changes is in the melting point. The addition of fluoride ion can lower the melting point of sodium monofluorophosphate. This is due to the disruption of the crystal lattice structure by the fluoride ion. As a result, the compound becomes more fluid at lower temperatures.
In terms of solubility, the fluoride ion can increase the solubility of sodium monofluorophosphate. This is because the fluoride ion can interact with water molecules, making it easier for the compound to dissolve. For example, a study found that the solubility of sodium monofluorophosphate in water increased by 20% when fluoride ion was present. This increased solubility is beneficial in dental care products as it allows for a higher concentration of the active ingredient to be delivered to the teeth.

In conclusion, the fluoride ion has a profound impact on the chemical properties and physical characteristics of sodium monofluorophosphate. Its influence on reactivity, stability, melting point, and solubility makes it an essential factor in the effectiveness of sodium monofluorophosphate in dental care and other applications.
III.Applications and Significance
Sodium Monofluorophosphate in Toothpaste
Toothpaste grade Sodium monofluorophosphate is widely used in toothpaste. As mentioned earlier, it acts as an anticaries agent and tooth desensitizer. Our toothpaste features naturally sourced sodium monofluorophosphate as its active ingredient to provide cavity protection, and calcium to clean. Research shows that toothpaste containing sodium monofluorophosphate can significantly reduce the incidence of tooth decay. For example, a study conducted over a period of two years found that children who used toothpaste with sodium monofluorophosphate had a 30% lower risk of developing cavities compared to those who used toothpaste without it.

Water Fluoridation
In addition to toothpaste, sodium monofluorophosphate is also used in water fluoridation. Water fluoridation is the process of adding fluoride to drinking water to prevent tooth decay. Sodium monofluorophosphate is one of the compounds used for this purpose. It helps in strengthening the enamel of teeth and reducing the risk of cavities. According to data, communities with fluoridated water have a lower incidence of tooth decay. For instance, a city that implemented water fluoridation saw a 40% reduction in the number of cavities among children within five years.
As a Preservative and Fungicide
Sodium monofluorophosphate can also act as a preservative and fungicide. It is used in some industries to prevent the growth of bacteria and fungi. For example, in the wood preservation industry, sodium monofluorophosphate is used to protect wood from decay caused by fungi and insects. In the food industry, it can be used as a preservative to extend the shelf life of certain products.
Importance for Various Industries
Understanding the interaction between fluoride ion and sodium monofluorophosphate is crucial for various industries. In the dental care industry, it helps in developing more effective toothpaste and oral care products. In the water treatment industry, it enables the optimization of water fluoridation processes. For the wood and food industries, it aids in the selection and application of appropriate preservatives. Overall, this understanding can lead to improved products and processes, and better protection of public health.

IV.Conclusion
In conclusion, the fluoride ion has a profound and multifaceted influence on sodium monofluorophosphate. It plays a crucial role in determining the chemical properties of sodium monofluorophosphate, enhancing its stability and affecting its reactivity. The fluoride ion also has a significant impact on the physical characteristics of sodium monofluorophosphate, such as lowering its melting point and increasing its solubility.
The significance of this interaction extends to various fields. In the dental care industry, sodium monofluorophosphate is a key ingredient in toothpaste and other oral care products, helping to prevent tooth decay and desensitize teeth. Research has shown that toothpaste containing sodium monofluorophosphate can significantly reduce the incidence of cavities. In water fluoridation, sodium monofluorophosphate is used to strengthen the enamel of teeth and reduce the risk of cavities in communities. Additionally, it can act as a preservative and fungicide in industries such as wood preservation and the food industry.
Understanding the relationship between fluoride ion and sodium monofluorophosphate is essential for the development of more effective products and processes in these industries. It can lead to improved dental care, better water treatment, and enhanced preservation of materials. As research continues, we can expect further insights into the complex interaction between fluoride ion and sodium monofluorophosphate, opening up new possibilities for its application in different fields.
Besides the fluoride ion, the chemical is also effected by the phosphate ion it contains, and these ions form the unique sodium monofluorophosphate mechanism of action.
Related inquiry: