I. Introduction
In this article, we will explore the fascinating process of making sodium carbonate from sodium bicarbonate, delving into the chemistry, methods, and practical considerations involved. Whether you are a chemistry enthusiast, a student, or simply curious about the substances that shape our modern world, join us on this journey to uncover the secrets of sodium carbonate production.
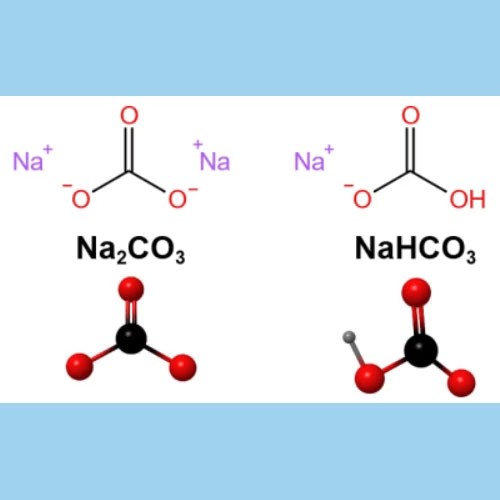
A. Sodium Bicarbonate: The Starting Material
Sodium bicarbonate, commonly known as baking soda or bicarbonate of soda, has the chemical formula NaHCO3. It is a white, crystalline powder with a slightly salty and alkaline taste. One of its most recognizable properties is its ability to react with acids, producing carbon dioxide gas.
For more details about its properties and uses, please read “what is sodium bicarbonate”.
B. Sodium Carbonate: The Desired Product
Sodium carbonate, with the chemical formula Na2CO3, is a white, odorless powder that is hygroscopic, meaning it readily absorbs moisture from the air. It is highly soluble in water, forming an alkaline solution. This alkaline nature is one of its most crucial characteristics, endowing it with a wide range of applications,such as glass, detergent,textile,chemical industries.
II. The Transformation Process
A. Thermal Decomposition: The Key Reaction
The conversion of sodium bicarbonate to sodium carbonate hinges on a fundamental thermal decomposition reaction.
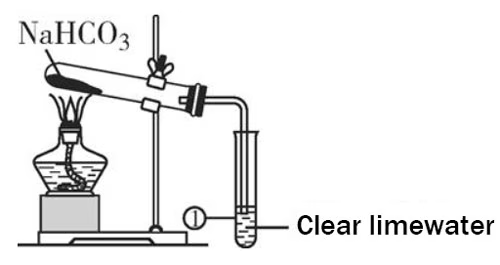
When heated, sodium bicarbonate undergoes a significant transformation. The chemical principle behind this lies in the instability of the bicarbonate ion (HCO₃⁻) under the influence of heat. As the temperature rises, the bicarbonate ion breaks down, releasing carbon dioxide (CO2) and water (H2O) in the form of vapor, leaving behind sodium carbonate (Na2CO3).
The balanced chemical equation for this reaction is:
2NaHCO₃ → Na₂CO₃ + H₂O + CO₂↑.
This equation beautifully illustrates the molecular changes that occur.
- Initially, two molecules of sodium bicarbonate react.
- The heat energy causes the bonds within the bicarbonate ions to rearrange.
- The hydrogen atom combines with the oxygen atom from another bicarbonate ion to form water, while the carbon dioxide is liberated.
- What remains is one molecule of sodium carbonate, which has a more stable structure compared to sodium bicarbonate.
B. Step-by-Step Procedure
1,Weighting
The first crucial step in the process is accurately weighing the required amount of sodium bicarbonate. This should be done using a precision balance. The amount needed depends on the scale of the experiment or the desired quantity of sodium carbonate.

For a small-scale laboratory experiment, a few grams might suffice.
For larger demonstrations or if a significant amount of sodium carbonate is needed, the quantity can be adjusted accordingly.
2,Heating
Once the sodium bicarbonate is weighed, it is carefully placed in the crucible. The crucible is then placed on the tripod stand above the burner.
Ensure that the crucible is clean and dry to prevent any unwanted reactions or impurities. Begin heating gradually. It is important not to apply high heat immediately as this could cause the sodium bicarbonate to spatter, leading to loss of material and inaccurate results. Start with a low flame and slowly increase the temperature while continuously monitoring the thermometer.

3,Monitering the gas
As the heating progresses, the reaction begins. The sodium bicarbonate will start to decompose, and you will notice the release of vapor, which is a combination of water and carbon dioxide.
Monitor the reaction progress closely. The color and texture of the substance in the crucible will change.
Initially, the white crystalline sodium bicarbonate will start to lose its crystalline structure and become more powdery. Continue heating until the evolution of gas ceases. This indicates that the decomposition is nearing completion. To ensure complete decomposition, maintain the temperature at the appropriate level for a few more minutes.
4, Complete experiment
Turn off the burner and allow the crucible to cool down to room temperature. The resulting solid in the crucible is now sodium carbonate, ready for further use or analysis.
III. DIY and Household Uses
For home enthusiasts interested in DIY projects, the knowledge of converting sodium bicarbonate to sodium carbonate can come in handy. In homemade cleaning agents, sodium carbonate can be a powerful ingredient. It can be used to make a heavy-duty cleaner for greasy stovetops or dirty ovens. By mixing sodium carbonate with a small amount of water to form a paste, it can cut through tough grease and grime, leaving surfaces sparkling clean. It is also effective in removing stains from clothing. When added to the laundry detergent, it boosts the cleaning power, especially for heavily soiled items. To know more about usage of sodium carbonate, please check related article: Sodium carbonate vs sodium bicarbonate.

IV. Troubleshooting and FAQs
A. Common Problems Faced
One of the most frequently encountered issues is incomplete decomposition of sodium bicarbonate. This can manifest as a residue that still fizzes when an acid is added, indicating the presence of unreacted bicarbonate. It often occurs when the heating temperature is too low or the reaction time is insufficient.
Another problem is uneven heating, which can lead to inconsistent product quality. Some parts of the sample may fully decompose while others remain underreacted, resulting in a mixture of sodium carbonate and sodium bicarbonate. This is typically caused by rapid heating or improper placement of the crucible in the heat source.
Product contamination is also a concern. Impurities can enter the final sodium carbonate, either from the starting sodium bicarbonate or due to unclean equipment. These impurities can affect the performance of the sodium carbonate in its intended applications, such as reducing the cleaning efficacy in detergents or causing defects in glass manufacturing.
B. Solutions and Tips
To address incomplete decomposition, ensure that the heating temperature reaches the optimal range and maintain it for an adequate duration. Use a reliable thermometer to monitor the temperature accurately. If in doubt, extend the heating time slightly while closely observing the reaction progress.
For uneven heating, start with a low flame and gradually increase the temperature, allowing the heat to distribute evenly throughout the sample. Stir the sodium bicarbonate gently (if possible) during the initial stages of heating to promote uniform heat transfer.
To prevent product contamination, always use high-quality, pure sodium bicarbonate and meticulously clean all equipment before use. After the reaction, consider conducting a simple purity test, such as testing the pH of a solution made from the produced sodium carbonate. If it deviates significantly from the expected pH for pure sodium carbonate, further purification steps like recrystallization may be necessary. Additionally, store the sodium carbonate in a sealed container to prevent it from absorbing moisture and other contaminants from the air, ensuring its long-term usability and quality.

