What is Sodium Bicarbonate?
Sodium bicarbonate, also known as baking soda, is a chemical compound with the formula NaHCO3. Its name is derived from the fact that it is a bicarbonate salt of sodium. In English, it is commonly referred to as “sodium bicarbonate”, while the term “baking soda” is widely used in everyday language.
As an acid – salt, it is formed by the neutralization of a strong base (sodium hydroxide, NaOH) with a weak acid (carbonic acid, H2CO3). The chemical reaction can be written as follows:
NaOH + H₂CO₃ → NaHCO₃ + H₂O.

The chemical structure of sodium bicarbonate consists of a sodium ion (Na⁺) and a bicarbonate ion (HCO₃⁻). This structure gives it certain chemical properties. For example, it can react with acids to produce carbon dioxide gas.
In a humid environment, sodium bicarbonate can absorb moisture from the air. When heated, it decomposes into sodium carbonate (Na2CO3), water (H2O), and carbon dioxide (CO2). The decomposition reaction is as follows:
2NaHCO₃ → Na₂CO₃ + H₂O + CO₂.
This decomposition property makes it useful in many applications. For instance, in baking, when baking soda is heated, it releases carbon dioxide gas, which causes dough to rise. In the food industry, it is used as a leavening agent in baking products.
The fact that it is relatively stable in normal conditions but decomposes under heat and in the presence of acids makes it a versatile compound. It can be used in a wide range of applications, from cooking and baking to industrial processes.
Sodium bicarbonate is a unique compound with distinct chemical properties. Its ability to react with acids, absorb moisture, and decompose under certain conditions makes it an important substance in various fields. It is a common ingredient in many household products and has significant industrial and commercial applications.
Physical Properties of Sodium Bicarbonate
Appearance
Sodium bicarbonate is a white crystalline powder or block shaped material with no odor. In its pure form, it appears as a fine, white powder. This powder is often free-flowing and can be easily handled. The white color is a characteristic feature that makes it distinguishable from other substances. It is commonly used in various applications, such as baking, where its white color is aesthetically pleasing.

Crystal Structure
The crystal structure of sodium bicarbonate is monoclinic. It consists of a lattice of sodium ions (Na⁺) and bicarbonate ions (HCO₃⁻). The lattice arrangement gives the crystal a specific shape and orientation. The crystal structure is important for understanding its physical properties and behavior. For example, the way the ions are arranged affects the density and hardness of the crystal.
Particle size
The particle size of sodium bicarbonate can vary depending on its production and intended use. Generally, it has an average particle size in the range of 15 – 300 μm. The smaller particles are more easily dispersed and can be used in applications where a fine powder is required, such as in baking or in the production of pharmaceuticals.
Solubility
Sodium bicarbonate is highly soluble in water. At 20 °C, its solubility is about 96 g/L. This means that in100 mL of water at 20 °C, approximately 9.6 g of sodium bicarbonate can dissolve. As the temperature increases, the solubility also increases. For example, at 60 °C, the solubility is around 165 g/L. This property makes it easy to dissolve in water, which is important for its use in various applications.
Melting and boiling point

The melting point of sodium bicarbonate is around 50 °C. At this temperature, it begins to decompose into sodium carbonate (Na2CO3), water (H2O), and carbon dioxide (CO2). The decomposition process is endothermic, meaning it absorbs heat. The boiling point of sodium b -icarbonate is not strictly a true boiling point in the traditional sense because it decomposes before reaching a boiling state. As it decomposes, it releases carbon dioxide gas, which is a characteristic feature of its thermal behavior.
Steam pressure
The steam pressure of sodium bicarbonate is relatively low. Since it decomposes upon heating, it does not have a significant vapor pressure at normal temperatures. This is in contrast to some other substances that can vaporize or have a significant vapor pressure. The low steam pressure is related to its stability and the fact that it does not easily convert into a gaseous state under normal conditions.
Chemical Properties of Sodium Bicarbonate
Thermal Stability
Sodium bicarbonate is thermally unstable. It begins to decompose at around 50 °C. As the temperature rises, it continues to break down and completely decomposes at 270 °C. The decomposition reaction of sodium bicarbonate is given by the equation:
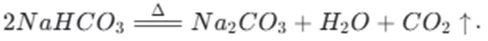
In contrast, sodium carbonate is much more stable. It does not decompose upon heating under normal conditions. This difference in thermal stability can be used to distinguish between sodium bicarbonate and sodium carbonate. For example, if we heat a sample of sodium bicarbonate, we will observe the release of carbon dioxide gas, which can be detected by passing the gas through limewater. The limewater will turn milky due to the formation of calcium carbonate. On the other hand, heating sodium carbonate will not produce such a reaction.

Reaction with Acids
When sodium bicarbonate reacts with acids, it undergoes a chemical reaction. For instance, in the human body, sodium bicarbonate reacts with hydrochloric acid. The reaction can be represented by the following equation:
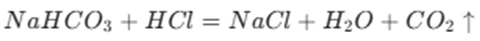
The ionic equation for this reaction is
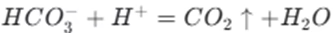
This reaction is widely used in the food industry. For example, baking soda (sodium bicarbonate) is used in baking. When it reacts with acidic substances in the dough, carbon dioxide gas is produced, causing the dough to rise. In the medical field, sodium bicarbonate is used to neutralize excess acid in the stomach. It can also be used to treat acid indigestion and heartburn.
Reaction with Bases
The reaction between sodium bicarbonate and a base is a form of neutralization. When sodium bicarbonate reacts with calcium hydroxide , the reaction depends on the amount of sodium bicarbonate present.
If sodium bicarbonate is in excess, the reaction is as follows:
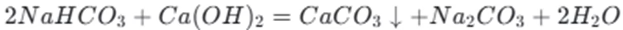
In this case, the bicarbonate ions react with the hydroxide ions from the calcium hydroxide.
If sodium bicarbonate is in short supply, the reaction is
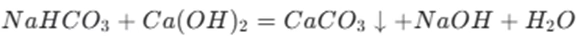
Here, the bicarbonate ion reacts with the hydroxide ion to form a carbonate ion and water.
Reaction with Salts
Sodium bicarbonate can react with certain salts. For example, when it reacts with aluminum chloride, it undergoes a hydrolysis reaction. The reaction can be represented by the following ionic equation:
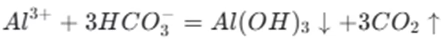
This reaction is important in chemical experiments and industrial processes. In the manufacturing of certain types of fire extinguishers, the reaction between sodium bicarbonate and aluminum chloride is used to produce carbon dioxide gas. The carbon dioxide gas helps to extinguish the fire. In the industrial production of some metal salts, this reaction can be used to purify the products or to control the reaction conditions.

Epand reading: Is sodium bicarbonate acidic or basic?
Production Methods of Sodium Bicarbonate
Solvay Process
The Solvay process, also known as the ammonia – soda process, is a widely used method for the production of sodium carbonate and sodium bicarbonate.
Process
Initial steps: First, ammonia ( NH3) is dissolved in water to form ammonia water. Then, carbon dioxide (CO2 ) is bubbled into the ammonia – water solution. The reaction between ammonia and carbon dioxide results in the formation of ammonium bicarbonate ( NH4HCO3). The chemical equation for this reaction is .
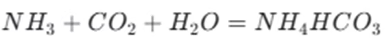
Formation of sodium bicarbonate: Next, sodium chloride ( NaCl) is added to the ammonium bicarbonate solution. Since sodium bicarbonate has a relatively low solubility in the solution, it precipitates out. The reaction is
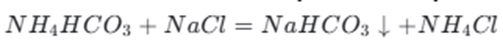
Recovery of ammonia: The ammonium chloride remaining in the solution can be treated with lime to regenerate ammonia. The reaction is
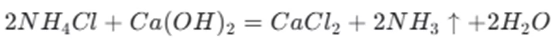

Advantages
Cost – effective: The raw materials such as sodium chloride, ammonia, and carbon dioxide are relatively inexpensive.
High – quality products: The process produces high – purity sodium bicarbonate and sodium carbonate.
Continuous production: It allows for continuous production, which is suitable for large – scale industrial operations.
Disadvantages
By – product issues: The production of calcium chloride as a by – product is a significant drawback. The disposal of calcium chloride can be costly and environmentally unfriendly.
Low efficiency: The overall efficiency of the process is relatively low, especially in terms of the utilization of sodium chloride.
Hou Debang’s improvement
The Hou Debang combined alkali production method,also known as the “Chinese soda – ash process”, was developed based on the Solvay process. In the Solvay process, the calcium chloride by – product is not efficiently utilized. In the Hou Debang method, instead of using calcium carbonate to generate carbon dioxide for the reaction, the ammonia – rich gas from the synthesis of ammonia is used directly. This not only reduces the amount of calcium chloride waste but also increases the overall efficiency of the process. The main advantage of the Hou Debang method is that it can produce both sodium carbonate and ammonium chloride simultaneously, without the need for additional calcium – based by – products.
Laboratory Preparation methods

The laboratory preparation of sodium bicarbonate can be carried out using the following steps:
Materials
Sodium carbonate (Na2CO3)
Carbon dioxide (CO2)
Water (H2O)
Procedure
Dissolution: First, dissolve sodium carbonate in water. The chemical equation for the dissolution of sodium carbonate in water is .
Reaction: Then, pass carbon dioxide gas through the solution. The reaction between sodium carbonate and carbon dioxide in water is .
Crystallization: As the reaction proceeds, the sodium bicarbonate gradually crystallizes out of the solution. The solution can be cooled to promote the crystallization process.
Operation key point
Controlled temperature: The temperature of the reaction should be carefully controlled. Generally, the reaction is carried out at room temperature or slightly higher.
Gas flow rate: The flow rate of carbon dioxide should be carefully controlled to ensure that the reaction proceeds smoothly.
Stirring: The solution should be stirred continuously to ensure uniform distribution of the reactants and promote the reaction.
Sodium Bicarbonate uses in Daily Life
In the Food Industry
Sodium bicarbonate plays a crucial role in the food industry as a leavening agent. When baking, it reacts with acidic components in the dough, such as cream of tartar or other organic acids. The reaction between sodium bicarbonate and these acids releases carbon dioxide gas. This gas gets trapped in the dough, creating numerous small bubbles, which cause the dough to expand and rise. For example, in the making of bread, the yeast produces carbon dioxide during fermentation, and sodium bicarbonate reacts with the acidic by – products of the yeast, further promoting the dough’s expansion.

The process of baking soda reacting with acid can be written as follows:
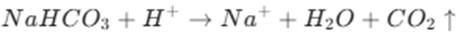
This reaction not only helps to create a light and fluffy texture in bread but also contributes to the characteristic flavor of the baked goods.
Common foods that use sodium bicarbonate include cakes, cookies, and biscuits. In cakes, for instance, the acidic ingredients in the batter react with sodium bicarbonate, producing carbon dioxide gas. This gas causes the cake to rise and gives it a soft and spongy texture.
It’s important to note that food – grade sodium bicarbonate is safe for consumption. However, excessive use can lead to a bitter taste in the food. For example, if too much sodium bicarbonate is used in a batch of cookies, the cookies may taste bitter due to the presence of unreacted sodium bicarbonate.
In the Medical field
In the medical field, sodium bicarbonate is used to neutralize excess acid in the stomach. The stomach contains hydrochloric acid, and when there is an over – production of acid, it can cause discomfort, heartburn, and indigestion. Sodium bicarbonate reacts with the hydrochloric acid in the stomach, neutralizing it and relieving these symptoms. The reaction can be represented as
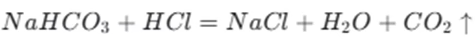
In addition to treating acid – related problems, sodium bicarbonate is also used in the treatment of metabolic acidosis. In certain medical conditions, such as diabetic ketoacidosis or kidney failure, the body’s acid – base balance is disrupted. Sodium bicarbonate can be used to correct this imbalance by increasing the alkalinity of the blood.

It can also be used to alkalize urine. This is important in cases where certain drugs or substances need to be excreted from the body. By alkalizing the urine, the solubility of these substances is increased, allowing them to be more easily excreted. For example, in the case of drug – induced kidney stones, sodium bicarbonate can be used to alkalize the urine, preventing the formation of stones, and promote their dissolution.
It’s important to note that while sodium bicarbonate can be beneficial in these situations, it should be used under the guidance of a medical professional. Over – dosage or improper use can lead to side effects such as nausea, vomiting, and in severe cases, even metabolic alkalosis.
In household cleaning
Sodium bicarbonate is a versatile and effective natural cleaning agent. It can be used to clean various surfaces in the home. For example, in the kitchen, it can be used to remove grease from stovetops and countertops. When mixed with water, sodium bicarbonate forms a paste that can be applied to greasy surfaces. The paste helps to break down the grease and make it easier to wipe away.

In the bathroom, it can be used to clean tiles and toilets. It can remove stains and odors effectively. For instance, when cleaning the toilet, sodium bicarbonate can be sprinkled into the bowl and left for a few minutes before flushing. This helps to remove stubborn stains and leave a fresh smell.
Another common use is in laundry. Sodium bicarbonate can be added to the laundry detergent to enhance its cleaning power. It can help to remove stains and odors from clothes, especially those caused by sweat or food spills.
Compared to chemical cleaning agents, sodium bicarbonate has several advantages. It is non – toxic, environmentally friendly, and biodegradable. It does not produce harmful fumes or residues, making it safe for both humans and the environment. Additionally, it is relatively inexpensive and readily available.
For example, instead of using harsh chemical cleaners, which may contain harmful substances, sodium bicarbonate can be used as a natural alternative. It can be used to clean kitchen appliances, such as ovens and microwave, without leaving behind any harmful residues.
Conclusion
In conclusion, sodium bicarbonate is a remarkable compound with a wide range of properties, production methods, and applications.
Properties
- Physical properties: Sodium bicarbonate is a white crystalline powder with no odor. Its crystal structure is monoclinic, and it has an average particle size of 15 – 300 μm. It is highly soluble in water, with a solubility of 96 g/L at 20 °C, and its melting point is around 50 °C.
- Chemical properties: It is thermally unstable, decomposing at around 50 °C, and completely decomposes at 270 °C. It reacts with acids, bases, and salts, and is involved in various chemical reactions.
Production methods

- Solvay Process: This method uses ammonia, carbon dioxide, and sodium chloride to produce sodium bicarbonate. It is cost – effective and can produce high – quality products. However, it has issues with by – products and low efficiency. The Hou debang improvement to the Solvay process addresses some of these problems by directly using ammonia – rich gas from ammonia synthesis, reducing waste and increasing efficiency.
- Laboratory preparation: In the laboratory, sodium bicarbonate can be prepared by dissolving sodium carbonate in water and then reacting with carbon dioxide. This method allows for controlled production and is suitable for small – scale experiments.
Applications
- Food industry: Sodium bicarbonate is widely used as a leavening agent in baking. It reacts with acidic components in dough, releasing carbon dioxide gas, which causes the dough to rise. It also contributes to the flavor and texture of baked goods.
- Medical field: It is used to neutralize excess acid in the stomach, treat metabolic acidosis, and alkalize urine. It is an important tool in treating various medical conditions related to acid – base balance.
- Household cleaning: Sodium bicarbonate is a versatile cleaning agent. It can remove grease, stains, and odors from various surfaces, and is environmentally friendly and non – toxic.
The importance of sodium bicarbonate cannot be overstated. It plays a crucial role in many aspects of our lives, from food preparation to medical treatment. Its unique properties make it suitable for a wide range of applications, and its availability and affordability make it accessible to many people.
There are several areas of research that could further enhance our understanding of sodium bicarbonate. For example, more research could be done on its use in new applications, such as its role in environmental protection and sustainable development. Additionally, improvements in production methods could lead to more efficient and cost – effective production of sodium bicarbonate.
As we continue to explore the world of chemistry, sodium bicarbonate will remain an important subject of study. Whether it’s used in the laboratory, in industry, or in our daily lives, its properties and applications will continue to be relevant and valuable. We encourage readers to further explore the world of sodium bicarbonate, and to discover its many potential uses and benefits.
Expand reading:

