I. Introduction
In our daily lives and industrial production, sodium carbonate and sodium bicarbonate are two commonly encountered chemical substances. You might have noticed the label of “sodium carbonate” on the back of the detergent bottle when doing household chores, and sodium bicarbonate is often seen in the kitchen as a leavening agent for making fluffy cakes. These two substances, though similar in name, have different properties and uses. Understanding their differences can not only help us make better use of them in daily life but also gain a deeper understanding of the magic of chemistry. So, let’s embark on a journey to explore the distinctions between sodium carbonate and sodium bicarbonate.
II. Basic Properties
A. Chemical Formulas and Molecular Weights
The chemical formula of sodium carbonate is Na2CO3, and its molecular weight is approximately 105.99 g/mol. It consists of two sodium ions (Na⁺) and one carbonate ion (CO₃²⁻). The carbonate ion is a polyatomic ion with a central carbon atom bonded to three oxygen atoms. In contrast, sodium bicarbonate has the chemical formula NaHCO3 and a molecular weight of around 84.01 g/mol. It contains one sodium ion (Na⁺), one hydrogen ion (H⁺), and one bicarbonate ion (HCO₃⁻). The bicarbonate ion is an amphiprotic species, which means it can act as both an acid and a base. This difference in chemical composition leads to distinct properties and behaviors of the two substances.
B. Physical Appearance
Sodium carbonate is typically a white, odorless powder. It can appear either crystalline or amorphous, depending on the method of preparation and its hydration state. Anhydrous sodium carbonate is a fine powder, while the hydrated forms, such as the decahydrate (Na2CO3·10H2O), are crystalline and resemble small, transparent crystals. In contrast, sodium bicarbonate is also white but has a finer, more powdery texture compared to sodium carbonate. It often appears as a fluffy, white powder with a crystalline structure that is less pronounced than that of hydrated sodium carbonate. When observed under a microscope, the crystals of sodium bicarbonate can be seen to have a characteristic shape, which can help distinguish it from other similar substances. Although it may be difficult to tell the difference between the two substances with the naked eye in some cases, sodium carbonate is generally more granular and compact, while sodium bicarbonate is softer and more voluminous.

C. Odor and Taste
Both sodium carbonate and sodium bicarbonate are odorless in their pure forms. However, when dissolved in water, they can impart a slightly alkaline odor, which is more noticeable in concentrated solutions. In terms of taste, sodium carbonate has a strong alkaline and bitter taste. It is not suitable for direct consumption due to its caustic nature, which can cause irritation to the mouth and digestive tract. Sodium bicarbonate, on the other hand, has a milder, slightly salty and alkaline taste. It is often used in cooking and baking, as it can enhance the flavor of certain foods without overpowering them. The difference in taste is due to the different degrees of alkalinity and the presence of the hydrogen ion in sodium bicarbonate, which affects its interaction with taste receptors on the tongue. In food applications, the subtle taste of sodium bicarbonate can contribute to the overall flavor profile, while sodium carbonate is mainly used in industrial processes where taste is not a concern.
III. Solubility and pH Levels
A. Solubility in Water
The solubility of sodium carbonate and sodium bicarbonate in water differs significantly. Sodium carbonate is highly soluble in water. At room temperature (around 25 °C), approximately 220 grams of sodium carbonate can dissolve in 1 liter of water, forming a clear solution. As the temperature increases, its solubility further rises. For example, at 100 °C, the solubility of sodium carbonate can reach up to about 450 grams per liter. This property makes it suitable for applications where a large amount of dissolved carbonate ions are needed, such as in water treatment processes to adjust the water hardness.

In contrast, sodium bicarbonate has a relatively lower solubility in water. At 20 °C, its solubility is only about 96 grams per liter. Moreover, when dissolved, sodium bicarbonate can undergo a slight decomposition reaction, especially at higher temperatures or in acidic environments, releasing carbon dioxide gas. This limited solubility and the tendency to decompose affect its usage in different scenarios. For instance, in some chemical reactions where a controlled release of carbon dioxide is required, the solubility characteristics of sodium bicarbonate can be exploited.
B. pH Values and Alkalinity
When dissolved in water, both sodium carbonate and sodium bicarbonate produce alkaline solutions due to the hydrolysis of their respective ions.
Sodium carbonate forms a relatively strong alkaline solution. A saturated solution of sodium carbonate typically has a pH value around 11.5, indicating a high concentration of hydroxide ions (OH⁻).
Sodium bicarbonate, on the other hand, results in a weakly alkaline solution. Its aqueous solution usually has a pH value in the range of 8.3 to 8.4. This mild alkalinity is beneficial in food applications.
IV. Thermal Stability
A. Decomposition Temperatures
When it comes to thermal stability, sodium carbonate and sodium bicarbonate exhibit significant differences. Sodium carbonate is relatively stable at high temperatures. It does not decompose readily and can withstand temperatures up to around 851 °C.
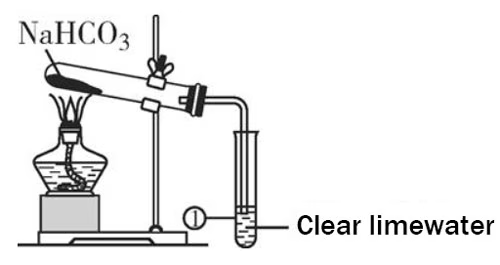
In contrast, sodium bicarbonate is much less thermally stable. It begins to decompose at around 50 °C, and the decomposition process is more pronounced as the temperature rises. By the time the temperature reaches 270 °C, the decomposition of sodium bicarbonate is complete.
The chemical equation for the decomposition of sodium bicarbonate is:
2NaHCO₃ → Na₂CO₃ + H₂O + CO₂↑.
This reaction is endothermic, meaning it absorbs heat as it progresses. As the temperature increases, the rate of carbon dioxide and water vapor generation accelerates, which can be observed as effervescence or bubbling if the sodium bicarbonate is in a confined space.
B. Practical Implications of Thermal Stability
The difference in thermal stability between sodium carbonate and sodium bicarbonate has profound practical implications.

In the field of baking, for example, sodium bicarbonate‘s ability to decompose at relatively low temperatures and release carbon dioxide gas is what makes it a popular leavening agent. When added to dough, as the dough is heated in the oven, the sodium bicarbonate decomposes, and the carbon dioxide gas gets trapped within the dough structure, causing it to rise and resulting in a light and fluffy texture. If sodium carbonate were used instead, due to its high thermal stability, it would not decompose under normal baking temperatures, and the dough would not rise in the same way, leading to a dense and unappealing final product.
In firefighting, sodium bicarbonate’s thermal decomposition property plays a vital role. It is used in some fire extinguishers, particularly those designed for Class B and C fires (involving flammable liquids and electrical equipment, respectively). When the extinguisher is activated and the sodium bicarbonate powder is discharged onto the fire, the heat causes it to decompose, releasing carbon dioxide. The carbon dioxide gas displaces oxygen, smothering the fire by reducing the oxygen concentration in the immediate vicinity of the flames. Sodium carbonate, with its high thermal stability, would not be suitable for this application as it would not release carbon dioxide readily upon exposure to the heat of the fire.

Thus, the thermal stability characteristics of these two substances are carefully considered to ensure their optimal use in various practical scenarios.
V. Chemical Reactivity
A. Reaction with Acids
Both sodium carbonate and sodium bicarbonate react vigorously with acids, but there are differences in their reaction rates and stoichiometry.
When sodium carbonate reacts with an acid, such as hydrochloric acid (HCl), the reaction proceeds as follows:
Na2CO3 + 2HCl → 2NaCl + H2O + CO2↑
Sodium bicarbonate, on the other hand, reacts with acids according to the equation:
NaHCO₃ + HCl → NaCl + H₂O + CO₂↑

B. Reaction with Bases
While both sodium carbonate and sodium bicarbonate are themselves bases, they can also react with stronger bases in certain situations. Sodium carbonate, when reacted with a strong base like sodium hydroxide (NaOH), does not undergo a typical acid-base neutralization reaction in the traditional sense. Instead, it can participate in an exchange reaction in aqueous solution. For example, in some industrial processes involving the adjustment of alkalinity, the addition of sodium carbonate to a solution with excess hydroxide ions can help buffer the pH and control the overall alkalinity.
Sodium bicarbonate, being amphiprotic, can react with bases as well. When reacted with a base, it acts as an acid and donates a proton. The reaction with sodium hydroxide can be represented as:
NaHCO₃ + NaOH → Na₂CO₃ + H₂O

In agriculture, the reaction of sodium bicarbonate with alkaline soils can also play a role in adjusting the soil pH. If the soil is too alkaline, the addition of appropriate amounts of sodium bicarbonate can help lower the pH slightly, making it more suitable for plant growth.

C. Reaction with Salts
Sodium carbonate and sodium bicarbonate can also react with certain salts in solution, leading to various chemical changes. For example, when sodium carbonate reacts with calcium chloride (CaCl2) in aqueous solution, a double displacement reaction occurs:
Na₂CO₃ + CaCl₂ → CaCO₃↓ + 2NaCl
In this reaction, calcium carbonate precipitates out of the solution as a white solid, while sodium chloride remains in the solution. This reaction is often used in water treatment to remove calcium ions from hard water. By adding sodium carbonate, the calcium ions are precipitated as calcium carbonate, which can then be filtered out, reducing the water hardness.
Sodium bicarbonate can also react with some salts. In the presence of aluminum sulfate (Al2(SO4)3), a reaction takes place in aqueous solution:
6NaHCO₃ + Al₂(SO₄)₃ → 2Al(OH)₃↓ + 3Na₂SO₄ + 6CO₂↑

Here, aluminum hydroxide precipitates, and carbon dioxide gas is released. This reaction is utilized in some chemical synthesis processes and in the treatment of certain types of industrial wastewater. The precipitation of aluminum hydroxide can help remove aluminum ions from the solution, and the release of carbon dioxide can have additional effects such as agitation or gas stripping in the treatment process. Overall, the reactions of sodium carbonate and sodium bicarbonate with salts offer diverse applications in different fields, from chemical manufacturing to environmental remediation.
VI. Industrial Applications
Sodium Carbonate:
Glass Manufacturing: The glass industry is the largest consumer of sodium carbonate. When making glass, sodium carbonate acts as a flux, lowering the melting point of silica (SiO2). This allows the glass to be formed at lower temperatures, reducing energy consumption during the manufacturing process. The sodium carbonate helps to dissolve impurities and promote the fusion of the materials, resulting in a homogeneous glass melt.
Paper Production: In the paper-making process, sodium carbonate is used in the pulping stage. It can improve the quality of the paper, making it stronger and more suitable for writing, printing, and other applications. Additionally, sodium carbonate can adjust the pH of the papermaking process, ensuring optimal conditions for the chemical reactions involved.
Metallurgy: In metallurgical operations, sodium carbonate serves multiple purposes. For example, in the smelting of iron and steel, it can act as a flux to remove impurities such as sulfur and phosphorus. In non-ferrous metallurgy, such as the extraction of aluminum, sodium carbonate is used in certain processes to adjust the electrolyte composition and improve the efficiency of the electrochemical reactions.

Sodium Bicarbonate:
Food Industry: Sodium bicarbonate is widely used in the food industry as a leavening agent. In baking, when it is combined with acidic ingredients like cream of tartar, yogurt, or vinegar, it undergoes a chemical reaction that releases carbon dioxide gas. It is also used in the production of carbonated beverages, where it provides the source of carbon dioxide to give the drinks their characteristic fizziness. In addition, sodium bicarbonate can act as a buffer to control the pH of food products, preventing spoilage and maintaining freshness.
Pharmaceutical Applications: In medicine, sodium bicarbonate has several uses. It is commonly used as an antacid to neutralize excess stomach acid. Sodium bicarbonate is also used in some intravenous solutions to correct acid-base imbalances in the body. In certain medical procedures, such as dialysis, it may be used to adjust the pH of the dialysis fluid.

Firefighting: Sodium bicarbonate is an important component in some types of fire extinguishers, particularly those designed for Class B and C fires. Class B fires involve flammable liquids like gasoline, oil, and paint, while Class C fires involve electrical equipment.
Related article: Unveiling the Wonders of Sodium Bicarbonate.
VII. Household and Daily Life Applications
A. In the Kitchen
In the kitchen, sodium carbonate and sodium bicarbonate have distinct yet equally valuable roles. Sodium carbonate, with its strong alkalinity, is often used for soaking and cleaning kitchen utensils, especially those with tough grease and burnt-on food residues. For example, when dealing with a greasy baking pan or a pot with caked-on food, a solution of sodium carbonate can be used to soak the item. The alkaline nature of the solution helps to break down the fats and oils, making them easier to scrub off. It can also be used to clean countertops, sinks, and stovetops, leaving them sparkling clean. However, it should be used with caution as its caustic nature can damage certain surfaces if not rinsed thoroughly.

Sodium bicarbonate, on the other hand, is a staple in baking. As mentioned earlier, it acts as a leavening agent when combined with acidic ingredients. Additionally, sodium bicarbonate can be used to neutralize odors in the refrigerator. Placing an open box of sodium bicarbonate in the fridge helps absorb unpleasant smells, keeping the interior fresh.
B. Cleaning and Hygiene
In the realm of cleaning and hygiene, both substances find extensive use. Sodium carbonate is a key ingredient in many laundry detergents. Its strong alkalinity helps to remove stains, especially those caused by oils, grease, and proteins. In a washing machine, the sodium carbonate in the detergent helps to break down dirt and keep the laundry clean. It can also be used as a pre-soak for heavily soiled clothes. For example, for clothes with grass stains or food grease, soaking them in a sodium carbonate solution before washing can enhance the cleaning effect. In addition, sodium carbonate can be used to clean bathroom tiles and floors. A solution of sodium carbonate can dissolve soap scum and mineral deposits, leaving the surfaces shiny and clean.

Sodium bicarbonate also has multiple applications in cleaning and personal care. In personal care, it can be used as a mild abrasive in toothpaste. The fine particles of sodium bicarbonate help to scrub away plaque and surface stains on teeth, while its mild alkalinity helps to neutralize acids in the mouth, providing a fresh feeling.
In household cleaning, it can be used to clean carpets. Sprinkling a small amount of sodium bicarbonate on a carpet, letting it sit for a while, and then vacuuming it up can help remove odors and freshen the carpet. It can also be used to clean delicate surfaces such as silverware. A paste made of sodium bicarbonate and water can be gently rubbed onto silver to remove tarnish without scratching the surface.
VIII. Conclusion
In conclusion, sodium carbonate and sodium bicarbonate, despite their similar names, possess distinct chemical and physical properties that lead to diverse applications in various fields. Sodium carbonate, with its higher alkalinity, solubility, and thermal stability, is predominantly used in industrial processes such as glass manufacturing, paper production, and metallurgy. On the other hand, sodium bicarbonate, with its milder alkalinity, lower thermal stability, and ability to release carbon dioxide upon heating or reaction with acids, finds extensive use in the food industry as a leavening agent, in medicine as an antacid, and in firefighting. Understanding these differences is not only crucial for professionals in relevant industries but also beneficial for everyday consumers to make the most of these substances in household chores, cooking, and cleaning.
Expand reading:
What’s the Reaction between Acetic Acid and Sodium Bicarbonate.

