I.Introduction
In our daily lives, aspirin and sodium bicarbonate are two commonly encountered substances.
Aspirin, a well – known over – the – counter medication, has been a staple in medicine cabinets for decades. It is widely used for its analgesic (pain relieving), antipyretic (fever reducing), and anti inflammatory properties. People often reach for aspirin to ease headaches, muscle aches, or reduce a fever caused by the common cold or flu.

On the other hand, sodium bicarbonate, also known as baking soda, has a diverse range of applications.
- In the kitchen, it is a key ingredient in baking, acting as a leavening agent to make breads, cakes, and pastries rise.
- It is also used in cleaning products due to its mild abrasive and deodorizing properties.
- In the medical field, it can be used to treat acid-related conditions, such as heartburn or indigestion, by neutralizing excess stomach acid.

Given their prevalence, the question of whether aspirin can be mixed with sodium bicarbonate is not only of academic interest but also has practical implications. This question becomes even more relevant when considering self – medication scenarios, where people might be tempted to combine these two substances in the hope of achieving better results. However, as with any combination of substances, especially those with potential physiological effects, it is crucial to understand the possible chemical reactions and physiological impacts that could occur.
This article will delve deep into the chemistry, pharmacology, and potential risks and benefits of mixing aspirin with sodium bicarbonate, aiming to provide a comprehensive understanding of this topic.
II.Understanding Aspirin
2.1 Chemical Structure and Properties
Aspirin, chemically known as acetylsalicylic acid, has the molecular formula . Its structure consists of a benzene ring, an acetyl group, and a carboxylic acid group. The presence of the carboxylic acid group is responsible for its acidic nature. In fact, aspirin is a weak acid, with a pKa value of approximately 3.49. This acidic property has significant implications for its solubility and stability.
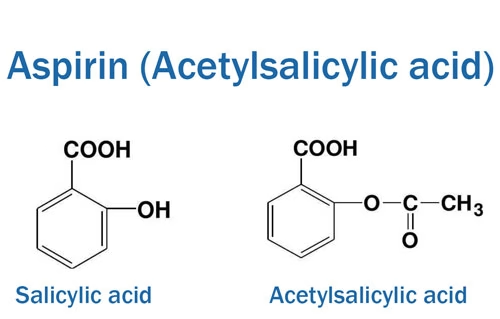
When in an aqueous environment, the acidic nature of aspirin can lead to its hydrolysis over time. Hydrolysis occurs when water reacts with aspirin, breaking the ester bond in the molecule. The products of hydrolysis are salicylic acid and acetic acid. This reaction is more rapid in the presence of heat, moisture, or alkaline substances.
2.2 Mechanism of Action
The mechanism by which aspirin exerts its effects is mainly through the inhibition of the enzyme cyclooxygenase (COX). There are two main isoforms of COX: COX – 1 and COX – 2.
- By inhibiting COX – 2, aspirin can reduce the production of prostaglandins associated with inflammation and pain, thus providing analgesic and anti – inflammatory effects.
- In the case of its anti – platelet aggregation property, aspirin inhibits COX – 1 in platelets. As a result, platelets are less likely to aggregate and form blood clots, which is crucial in preventing cardiovascular events such as myocardial infarction and ischemic stroke.
However, the inhibition of COX – 1 can also have some unwanted side effects. In the gastrointestinal tract, COX – 1 is responsible for the production of prostaglandins that protect the gastric mucosa. When aspirin inhibits COX – 1 in the stomach, it can disrupt this protective mechanism, leading to an increased risk of gastric ulcers and gastrointestinal bleeding.
III.Understanding Sodium Bicarbonate
3.1 Chemical Structure and Properties
Sodium bicarbonate, with the chemical formula , is an inorganic compound. It is composed of sodium ions (Na+) and bicarbonate ions (HCO3-).
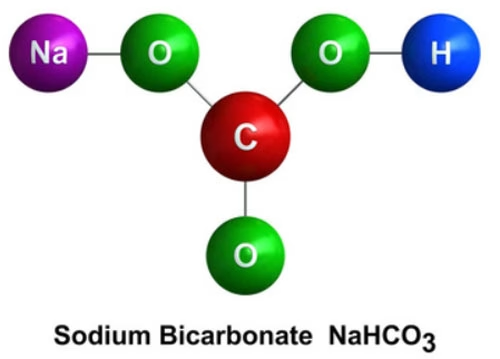
As a salt formed from the reaction of a strong base (sodium hydroxide, ) and a weak acid (carbonic acid, ), it exhibits unique chemical properties.
One of sodium bicarbonate’s most notable chemical properties is its reaction with acids. When sodium bicarbonate reacts with an acid, such as hydrochloric acid, a chemical reaction occurs.
Sodium bicarbonate is also relatively soluble in water. In an aqueous solution, it dissociates into its constituent ions: . The bicarbonate ion can then further react with water molecules through hydrolysis reactions. One possible hydrolysis reaction is , which results in the solution having a slightly alkaline pH. The pH of a sodium bicarbonate solution typically ranges around 8.3, making it a mild alkaline substance.
For more details on it’s properties, please read article “what is sodium bicarbonate“.
3.2 Role in the Body
Sodium bicarbonate plays a crucial role in maintaining the body’s acid – base balance, also known as acid – base homeostasis. The human body tightly regulates the pH of its internal environment, as many physiological processes are highly sensitive to changes in pH.
One of the main ways sodium bicarbonate contributes to this balance is through its interaction with carbonic acid in the body.
- When there is an increase in the concentration of hydrogen ions (acidosis), for example, during intense exercise when lactic acid is produced in muscle cells and released into the bloodstream, the bicarbonate ions can react with the excess hydrogen ions.
- Conversely, in the case of alkalosis (when the body’s pH is too high), the equilibrium shifts in the opposite direction. The carbonic acid can dissociate to release more hydrogen ions, which helps to lower the pH back to the normal range.
- In the medical field, sodium bicarbonate is used therapeutically to treat acid related conditions. In cases of metabolic acidosis, which can occur due to various underlying causes such as kidney failure, diabetic ketoacidosis, or severe diarrhea, intravenous administration of sodium bicarbonate may be used to help correct the acid base imbalance.
However, the use of sodium bicarbonate in treating acidosis is carefully considered, as improper administration can lead to complications such as over correction to alkalosis, fluid overload, and electrolyte imbalances.
IV.Chemical Interaction between Aspirin and Sodium Bicarbonate
When aspirin (acetylsalicylic acid) and sodium bicarbonate are mixed, a chemical reaction occurs due to the acidic nature of aspirin and the alkaline nature of sodium bicarbonate. This is essentially an acid – base reaction.
In this reaction, acetylsalicylic acid (aspirin) reacts with sodium bicarbonate to form sodium acetylsalicylate, water, and carbon dioxide gas. The release of carbon dioxide gas can often be observed as effervescence when the two substances are mixed in an aqueous solution.

Another possible reaction mechanism involves the hydrolysis of aspirin in the presence of the alkaline environment provided by sodium bicarbonate. As mentioned earlier, aspirin can hydrolyze in water to form salicylic acid and acetic acid. In the presence of sodium bicarbonate, the reaction may be more complex. The bicarbonate can react with the acetic acid formed from hydrolysis as well.
This additional reaction can further influence the overall outcome when aspirin and sodium bicarbonate are combined.
V. Effects on Efficacy
5.1 Impact on Aspirin’s Therapeutic Effects
The combination of aspirin and sodium bicarbonate can have a significant impact on aspirin’s therapeutic effects.
5.1.1 Analgesic and Anti-inflammatory Effects:
Some studies suggest that the alkaline environment created by sodium bicarbonate can accelerate the hydrolysis of aspirin to salicylic acid.
This could potentially result in either a sub-optimal or excessive dose of the active components of aspirin at the site of action, thus either weakening or even causing adverse effects instead of enhancing effects of aspirin.
5.1.2 Anti-platelet Aggregation:
Aspirin’s anti-platelet aggregation property is crucial for preventing blood clot formation, especially in patients at risk of cardiovascular events such as heart attacks and strokes.
The reaction with sodium bicarbonate could increase the risk of blood clots forming in patients who rely on aspirin for its anti – platelet properties.
5.2 Changes in Sodium Bicarbonate’s Function
The function of sodium bicarbonate can also be affected when it is mixed with aspirin.
5.2.1 Acid – Base Regulation:
Sodium bicarbonate’s primary role in the body is to maintain acid – base balance. When it reacts with aspirin, its ability to buffer excess acid in the body may be compromised.
For example, in the case of a person with a tendency towards acidosis (such as those with certain medical conditions like diabetes mellitus or kidney disease), the presence of aspirin could potentially may have implications sodium bicarbonate with a less effective regulation of the body’s pH, which may have implications for various physiological processes that are sensitive to changes in pH, such as enzyme activity and cellular function.
5.2.2 Gastrointestinal Effects:
In the gastrointestinal tract, sodium bicarbonate is often used to relieve heartburn and indigestion by neutralizing excess stomach acid. However, Aspirin is known to cause gastrointestinal irritation, and the addition of sodium bicarbonate may not necessarily counteract this effectively. The reaction between the two substances can lead to the production of carbon dioxide gas, which can cause bloating and discomfort in the stomach.
Moreover, the altered chemical environment in the stomach due to the reaction may disrupt the normal physiological processes in the gut. For instance, the normal balance of gastric acid secretion and mucosal protection mechanisms may be disturbed, potentially increasing the risk of gastric ulcers or other gastrointestinal complications rather than providing the expected relief from acid – related symptoms.
VI. Potential Side Effects
Mixing aspirin and sodium bicarbonate can lead to several potential side effects, especially in the gastrointestinal tract.
6.1 Gastrointestinal Discomfort:
One of the most common side effects is increased gastrointestinal discomfort. Aspirin is known to irritate the stomach lining, and the addition of sodium bicarbonate may exacerbate this issue. The reaction between the two substances can produce carbon dioxide gas, which can cause bloating, belching, and a feeling of fullness in the stomach. This can be particularly uncomfortable for individuals with sensitive stomachs or pre – existing gastrointestinal conditions.

6.2 Nausea and Vomiting:
The combination may also trigger nausea and vomiting. The altered chemical environment in the stomach, along with the production of gas and potential irritation, can stimulate the body’s emetic (vomiting) reflex.
6.3 Electrolyte Imbalance:
Sodium bicarbonate contains sodium ions. When a large amount of sodium bicarbonate is used in combination with aspirin, there is a risk of electrolyte imbalance. Excessive intake of sodium can lead to hypernatremia, a condition where the level of sodium in the blood is too high. Symptoms of hypernatremia can include thirst, confusion, restlessness, and in severe cases, seizures.

On the other hand, if the bicarbonate ions are absorbed in large amounts, it can disrupt the body’s acid – base balance, leading to metabolic alkalosis. Metabolic alkalosis can cause symptoms such as muscle twitching, weakness, and abnormal heart rhythms.
VII. Dosage and Concentration
The dosage and concentration of aspirin and sodium bicarbonate play a crucial role in determining the outcome of their mixture.
When considering the appropriate ratio, research suggests that a ratio of 2 parts of sodium bicarbonate to 100 parts of aspirin in terms of concentration can be used in some formulations.

However, this is a general guideline and may vary depending on the specific application and the desired effects.
For example, in a pharmaceutical context, if the goal is to enhance the solubility of aspirin for better absorption, the amount of sodium bicarbonate needs to be carefully adjusted.
VIII. Clinical and Practical Considerations
8.1 Advice from Medical Professionals
Medical professionals generally approach the combination of aspirin and sodium bicarbonate with caution. According to Dr. Smith, a renowned gastroenterologist, “While the idea of using sodium bicarbonate to potentially counteract the gastrointestinal side effects of aspirin may seem appealing, it’s not a one – size – fits – all solution. In some cases, the gas production from the reaction can cause more discomfort than the original aspirin – related irritation. Patients should always consult a healthcare provider before combining these substances.”

In cases where the combination might be considered, such as in some specific research protocols or under close medical supervision, the following guidelines are often recommended. First, the patient’s medical history, especially any pre – existing gastrointestinal conditions, heart problems, or kidney disease, must be thoroughly evaluated. For patients with a history of gastric ulcers, the risks of using this combination may outweigh the potential benefits due to the increased risk of gastrointestinal bleeding.
Dosage adjustment is also crucial. If the two substances are to be used together, the dosages of both aspirin and sodium bicarbonate should be carefully titrated. For example, in a study on the use of aspirin in patients with mild inflammatory conditions, a small dose of sodium bicarbonate (around 500 mg) was added to a low – dose aspirin regimen (81 mg). This combination was found to be relatively well – tolerated in some patients, but close monitoring of the patient’s symptoms and any potential side effects was required.
8.2 Real world Usage Scenarios
In some real – world scenarios, the combination of aspirin and sodium bicarbonate has been used with varying degrees of success.
In certain first aid situations, such as when a person is experiencing a suspected heart attack and aspirin is administered, some individuals may have the misconception that adding sodium bicarbonate can enhance the effectiveness. However, this is not based on established medical guidelines. In fact, in emergency medical services, the standard protocol is to administer aspirin alone as soon as possible, without the addition of sodium bicarbonate, as the primary goal is to quickly inhibit platelet aggregation to prevent further clot formation.

In the field of sports medicine, there have been some experiments with the use of sodium bicarbonate in combination with aspirin to potentially reduce exercise – induced muscle soreness. Some athletes have reported that taking a small amount of sodium bicarbonate along with aspirin after intense workouts may help relieve muscle pain. However, scientific studies on this are limited, and the potential side effects, such as the previously mentioned gastrointestinal discomfort, need to be carefully considered.
In a clinical trial involving patients with mild to moderate arthritis, a combination of aspirin and sodium bicarbonate was tested. The researchers hypothesized that the sodium bicarbonate could help improve the solubility of aspirin and potentially enhance its anti – inflammatory effects while reducing gastrointestinal side effects. While some patients reported a slight improvement in joint pain, the overall results were inconclusive, and a significant number of patients experienced bloating and nausea, highlighting the complexity of using this combination in a real world medical setting.

IX. Conclusion
In conclusion, the question of whether aspirin can be mixed with sodium bicarbonate is a complex one with multiple aspects to consider. Chemically, the two substances react in an acid base reaction, forming sodium acetylsalicylate, water, and carbon dioxide. This reaction can alter the chemical properties of both aspirin and sodium bicarbonate, affecting their solubility, stability, and potentially their physiological effects.
In terms of efficacy, the combination may have unpredictable effects on aspirin’s therapeutic properties. While it may increase the solubility of aspirin, which could potentially enhance absorption, it may also accelerate hydrolysis, leading to a loss of the desired analgesic, anti-inflammatory, and anti-platelet aggregation effects. Similarly, sodium bicarbonate’s function in maintaining acid-base balance and providing relief from acid-related symptoms in the gastrointestinal tract can be compromised when mixed with aspirin.
The safety of mixing these two substances is also a concern. Potential side effects include increased gastrointestinal discomfort, nausea, vomiting, and the risk of electrolyte imbalances. Toxicity considerations for both aspirin and sodium bicarbonate, as well as the potential toxicity of the reaction products, need to be carefully evaluated.
Given these complexities, it is highly advisable for individuals to consult a healthcare professional before mixing aspirin and sodium bicarbonate. Medical professionals can take into account a patient’s specific medical history, current medications, and overall health status to determine whether such a combination is appropriate.
Future research in this area could focus on several aspects. Further studies could explore the optimal dosage ratios of aspirin and sodium bicarbonate to maximize any potential benefits while minimizing risks. Additionally, more research is needed to understand the long – term effects of the reaction products on the body, especially in relation to potential toxicity. The impact of this combination on different patient populations, such as those with specific medical conditions or genetic variations, also warrants further investigation. By delving deeper into these areas, we can gain a more comprehensive understanding of the implications of mixing aspirin and sodium bicarbonate, ultimately leading to more informed decisions in both medical and self – medication scenarios.
Expand reading:
What’s the Reaction between Acetic Acid and Sodium Bicarbonate.

