I. Introduction
In the wonderful world of baking, achieving that perfect rise and fluffy texture is often the ultimate goal. Two common ingredients that play a crucial role in this process are baking powder and sodium bicarbonate. You might have found yourself in the kitchen, staring at a recipe, and wondering, “Can I use sodium bicarbonate instead of baking powder?” Well, you’re not alone, and this article is here to unravel the mystery.
II. Is baking powder bicarbonate sodium?
1. Baking Powder

Baking powder is a leavening agent, a crucial ingredient in the world of baking. It’s a combination of several components that work together to give our baked goods that desirable lift. To know types of baking powder and advantage of slow reaction baking powder, please read “sodium acid pyrophosphate baking powder”, from which we note the sodium bicarbonate is a part of baking powder. Beside sodium bicarbonate, an acid, and a filler (often cornstarch) are also necessary.
The baking powder is designed to release carbon dioxide gas when it comes into contact with moisture and heat. This gas gets trapped within the dough or batter, causing it to expand and creating those light, fluffy textures we love in cakes, muffins, biscuits, and more.
The production of baking powder involves carefully blending the right proportions of its components to ensure consistent performance. It’s then packaged to keep it dry and stable until use. Given its sensitivity to moisture, it’s essential to store baking powder in a cool, dry place in an airtight container. Once opened, it should be used within a reasonable time frame to guarantee its effectiveness.
2. Sodium bicarbonate

It is also known as baking soda, is a white crystalline powder with a slightly salty and alkaline taste. To know more about its other names, please read “what is the common name of sodium bicarbonate” and “why is sodium bicarbonate used for baking”.

3. The Key Differences
a. Chemical Composition
The fundamental disparity between baking powder and sodium bicarbonate lies in their chemical makeup.
- Baking powder is a carefully formulated blend. As mentioned earlier, it contains sodium bicarbonate as the base, which provides the potential for carbon dioxide generation. However, it also incorporates an acid, such as cream of tartar (potassium bitartrate), monocalcium phosphate, or sodium aluminum phosphate, depending on the type of baking powder. The acid is crucial as it reacts with the sodium bicarbonate under the right conditions to release carbon dioxide( Reference articel “What’s the Reaction between Acetic Acid and Sodium Bicarbonate”). Additionally, cornstarch is added as a filler. The cornstarch serves multiple purposes. It helps to keep the other components dry and separate, preventing premature reactions. It also dilutes the potency of the leavening agents slightly, ensuring a more controlled and even release of gas.
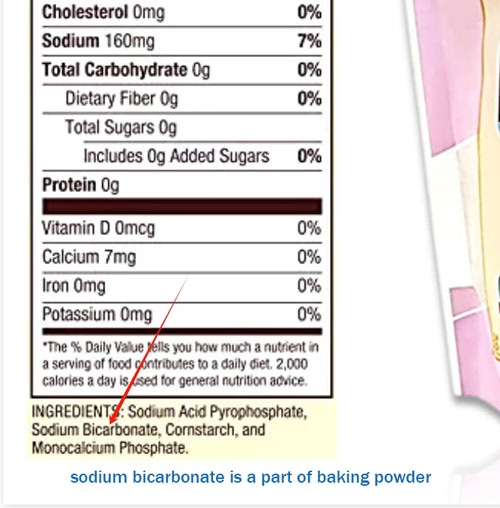
- In contrast, sodium bicarbonate doesn’t have the built-in acid component like baking powder. This simplicity means that it needs an external source of acid to fully function as a leavening agent. Without that acid, it won’t produce carbon dioxide in the same efficient and controlled manner as baking powder.
b. Reaction Mechanism
Baking powder is designed for a two-stage reaction.
- When it first comes into contact with moisture, whether it’s from milk, water, or eggs in a recipe, a portion of the sodium bicarbonate reacts with the acid present in the powder. This initial reaction releases a small amount of carbon dioxide. It’s like a gentle wake-up call for the batter or dough, starting the expansion process.
- As the baking progresses and the temperature rises, the heat triggers a second, more vigorous reaction. The remaining sodium bicarbonate reacts further with the acid, producing a larger volume of carbon dioxide.

This dual-action is what gives baked goods that consistent and desirable rise. The gas bubbles get trapped within the gluten network of the dough or the structure of the batter, creating those light and airy textures.
Sodium bicarbonate, on the other hand, requires either heat or an acid to react.
If you rely solely on heat, it starts to decompose at around 50-60°C (122-140°F). This decomposition breaks it down into sodium carbonate, water, and carbon dioxide. However, the resulting sodium carbonate is alkaline and can affect the taste and color of the baked product. When combined with an acid, such as lemon juice or buttermilk, it reacts immediately to produce carbon dioxide. But here’s the catch: you need to ensure the right balance of acid and sodium bicarbonate. Too much acid can lead to an overly acidic taste, while too little won’t fully utilize the leavening potential of the sodium bicarbonate.

c. Taste and Residue
Sodium bicarbonate has a distinctively alkaline taste.
If not used correctly and in the right proportions, it can leave behind a soapy or bitter aftertaste in the baked item. This is because when it decomposes or reacts incompletely, the residual sodium carbonate or unreacted sodium bicarbonate can impart that unpleasant flavor.
Moreover, the alkaline nature of sodium bicarbonate can cause baked goods to turn a darker color than intended. For example, cookies made with too much sodium bicarbonate might end up looking overly browned and have a tough texture.

Baking powder, when used as per the recipe instructions, doesn’t leave behind such a strong alkaline residue.
Since it contains the right balance of acid and base, the reaction is more complete, and the by-products are less likely to affect the taste and appearance of the final product. The flavors of the other ingredients in the recipe can shine through, and the baked good achieves the desired color and tenderness.
So, we could find sodium bicarbonate and baking powder are not interchangeable in all recipes. Baking soda needs an acid in the recipe to react and produce carbon dioxide, whereas baking powder already contains the necessary acid components and is formulated for a more balanced and predictable leavening effect. Understanding these differences is key to successful baking.
III. When Can You Substitute?
1. Recipes Requiring Little Rise
There are certain recipes where using sodium bicarbonate instead of baking powder can work quite well. If you’re making something that doesn’t rely heavily on a significant rise, like crackers or pancakes, you can make the substitution. For example, in a simple butter cracker recipe, the main goal is to achieve a crisp texture rather than a lofty, airy one. Replacing the baking powder with sodium bicarbonate won’t have a drastic negative impact. In fact, it might even give the crackers a slightly different, yet pleasant crunch.

When making pancakes, a small amount of sodium bicarbonate can be used. However, it’s important to note that you might need to adjust the recipe slightly. Since sodium bicarbonate reacts with acids, if your pancake batter contains acidic ingredients like buttermilk or yogurt, it can work in harmony. But if you’re using a basic milk-based batter, you might want to add a touch of lemon juice or vinegar to activate the sodium bicarbonate and get that gentle lift.

2. Adjusting for Acidity
One of the crucial aspects of substituting sodium bicarbonate for baking powder is accounting for acidity. As we know, baking powder already contains an acid component, while sodium bicarbonate needs an external acid source. In recipes where you decide to make the switch, you’ll need to introduce acidic ingredients.
For instance, if you’re making a cake and substituting baking powder with sodium bicarbonate, you could add a tablespoon of lemon juice or white vinegar to the batter. This added acid will react with the sodium bicarbonate, producing the necessary carbon dioxide for leavening.

Another option is to use buttermilk instead of regular milk. Buttermilk has a natural acidity that pairs well with sodium bicarbonate. If a recipe calls for 1 cup of milk, replacing it with 1 cup of buttermilk and adjusting the amount of sodium bicarbonate accordingly can yield good results.
It’s important to find the right balance. Too much acid can make the final product taste overly sour, while too little won’t fully activate the sodium bicarbonate. Experimenting with small batches and making notes of the changes you make is a great way to master this substitution technique.
IV. When It’s Not Advisable
1. Delicate Cakes and Pastries
When it comes to more delicate baked creations like chiffon cakes or sponge cakes, using sodium bicarbonate as a direct substitute for baking powder can be a recipe for disaster. These cakes rely on a precise and gentle leavening process to achieve their characteristic light and airy texture. Baking powder, with its controlled two-stage reaction, provides a steady stream of carbon dioxide that helps the batter expand evenly.

Sodium bicarbonate, on the other hand, can be a bit too aggressive. Without the proper balance of acid and the controlled release mechanism of baking powder, it can cause the cake to rise too quickly in some areas and not enough in others. This uneven rise often leads to a cake that collapses in the middle, resulting in a dense and unappealing texture. The delicate crumb structure that makes these cakes so desirable can be completely lost, turning a potentially beautiful dessert into a baking disappointment.

2. Recipes with Specific Ingredient Ratios
Some recipes are formulated with very specific ratios of ingredients, and any alteration can disrupt the delicate chemical balance. Take, for example, a classic angel food cake. This cake typically uses a large amount of egg whites, which provide structure and stability. The small amount of baking powder used is carefully calibrated to work in harmony with the egg whites and other ingredients to create a tall, fluffy cake with a tender interior.

If you were to substitute sodium bicarbonate, you’d not only need to account for the lack of built-in acid but also the potential impact on the overall texture. The stronger alkalinity of sodium bicarbonate could interact with the proteins in the egg whites in an unexpected way, causing them to denature or lose their ability to hold air. This could lead to a cake that doesn’t rise properly, has a rubbery texture, and fails to achieve the iconic height and softness that angel food cake enthusiasts expect. It’s a prime example of how a seemingly simple substitution can have far-reaching consequences in the complex world of baking chemistry.
V. Tips for Successful Substitution
1. Measuring Accurately
Accurate measurement is the cornerstone of a successful baking substitution. When using sodium bicarbonate instead of baking powder, precision becomes even more crucial. Since sodium bicarbonate is a more potent leavening agent in the right circumstances, even a slight over-measurement can lead to undesirable results.
Invest in a good set of measuring spoons and cups. For small amounts of sodium bicarbonate, a teaspoon or half-teaspoon measure is often sufficient. Make sure to level off the powder with a straight edge, like the back of a knife, to ensure you’re getting the exact amount called for in the recipe.

Typically, if a recipe calls for 1 teaspoon of baking powder, you might only need about ¼ to ½ teaspoon of sodium bicarbonate, depending on the other ingredients and the desired rise. This is because baking powder already has the acid component measured in, while with sodium bicarbonate, you need to balance it with any acids in the recipe. Keeping a conversion chart handy in the kitchen can be a great help until you become familiar with the ratios.

2. Mixing and Baking Techniques
Mixing plays a vital role when substituting sodium bicarbonate. First, make sure to thoroughly mix the sodium bicarbonate with the dry ingredients. This ensures an even distribution, preventing any pockets of concentrated leavening agent that could cause over-expansion in some areas and under-expansion in others.
When adding the wet ingredients, do so gently and mix just until combined. Over-mixing can lead to the premature release of carbon dioxide, resulting in a flatter final product. If you’re using an acid like lemon juice or vinegar to activate the sodium bicarbonate, add it to the wet ingredients first and then combine with the dry mixture.
In terms of baking, pay close attention to the temperature and time.

Since sodium bicarbonate can react more quickly than baking powder, you might need to adjust the baking temperature slightly lower and keep a closer eye on the baking progress. This helps to ensure that the gas release is controlled and the baked good rises evenly. For example, if a recipe usually bakes at 350°F (177°C) with baking powder, you could try reducing the temperature to 325°F (163°C) when using sodium bicarbonate and check for doneness a few minutes earlier than usual. Using an oven thermometer to monitor the actual oven temperature is also a good practice, as oven temperatures can sometimes vary from the set dial.
VI. Conclusion
In conclusion, while baking powder and sodium bicarbonate both play vital roles in baking as leavening agents, they are not one and the same. Baking powder offers a convenient, all-in-one solution with its built-in acid and controlled two-stage reaction, making it ideal for a wide variety of baked goods, especially those requiring a delicate and consistent rise. On the other hand, sodium bicarbonate, with its pure and potent nature, can be a great substitute in certain situations, provided you understand its need for an external acid source and adjust your recipe accordingly.
Whether you’re a novice baker looking to experiment or an experienced chef trying to make do with what’s in the pantry, don’t be afraid to try substituting sodium bicarbonate for baking powder. Just remember to take into account the recipe type, acidity levels, and follow the tips for accurate measurement and proper mixing and baking techniques. With a bit of practice and a willingness to learn from your baking adventures, you can successfully navigate the world of baking substitutions and create delicious treats that are sure to impress. So, put on your apron, preheat that oven, and let the baking experiments begin!

