Sodium bicarbonate, also known by its common name baking soda, has the chemical formula NaHCO3. It appears as a white, crystalline powder that is odorless and has a slightly alkaline taste. This compound is a salt formed by the neutralization of a strong base (sodium hydroxide) with a weak acid (carbonic acid). It is highly soluble in water, and when dissolved, it imparts a weak alkaline property to the solution. For more details, please read ” what is Sodium Bicarbonate “?
Understanding Kidney Stones
Kidney stones, also known as renal calculi, are solid masses that form within the kidneys. These stones develop when substances in the urine, such as calcium, oxalate, phosphate, and uric acid, become overly concentrated and precipitate out of the solution. This can occur due to a variety of factors, including dehydration, which reduces the volume of urine and causes solutes to accumulate; dietary habits rich in certain substances like oxalate-containing foods (e.g., spinach, rhubarb); metabolic disorders that alter the body’s normal handling of minerals and acids; and genetic predispositions that make some individuals more likely to form stones.
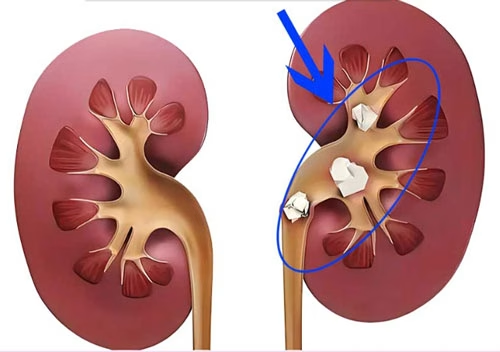
The composition of kidney stones can vary widely. Calcium oxalate stones are by far the most common, accounting for about 70-80% of all cases. These stones form when there is an excess of oxalate in the urine, which combines with calcium to create the hard, crystalline deposits. Calcium phosphate stones are another significant type, often associated with conditions that increase the urinary phosphate levels or alkalinity. Uric acid stones tend to develop in people with high levels of uric acid in their blood, which can result from diets rich in purines (found in organ meats, shellfish, and some alcoholic beverages) or certain medical conditions like gout. Struvite stones are less common and are typically linked to urinary tract infections caused by specific bacteria that produce enzymes promoting the formation of these stones.

When a kidney stone starts to move within the urinary tract, it can cause a range of distressing symptoms. The most characteristic symptom is severe pain, often described as colicky, which can radiate from the flank (side of the abdomen) to the groin area as the stone travels down the ureter. The pain may come in waves and can be so intense that it causes nausea, vomiting, and restlessness. Hematuria, or blood in the urine, is another common sign, which can range from microscopic amounts only detectable by a urine test to visibly pink or red urine. Additionally, patients may experience a frequent urge to urinate, a burning sensation during urination, or even difficulty passing urine if the stone obstructs the flow. In some cases, if the stone causes a complete blockage and leads to urinary tract infection or hydronephrosis (swelling of the kidney due to backed-up urine), more serious complications can ensue, potentially affecting kidney function. Understanding the nature and causes of kidney stones is crucial for exploring effective prevention and treatment strategies, such as the potential role of substances like sodium bicarbonate.
The Mechanism: Can It Really Dissolve Kidney Stones?
Theoretically, the potential of sodium bicarbonate to dissolve kidney stones lies in its alkaline nature (For more details please read ” is sodium bicarbonate acidic or basic “). When ingested, it can increase the alkalinity of urine. This change in pH is significant because many kidney stones, particularly those composed of uric acid, are more soluble in alkaline environments. Uric acid stones form when the urine is too acidic, causing uric acid to precipitate out and crystallize. By raising the urine pH with sodium bicarbonate, the equilibrium of the uric acid dissolution-precipitation process can be shifted, favoring dissolution.
For example, consider a patient with a history of recurrent uric acid kidney stones. Their urine is consistently acidic, leading to stone formation. When prescribed sodium bicarbonate, the alkalinity of the urine increases. This alteration makes the uric acid in the existing stones more likely to dissolve back into the urine, gradually reducing the size of the stones.
However, the reality is far more complex. Calcium-based stones, which are the most prevalent, do not respond as favorably. While sodium bicarbonate can change the urine pH, it may not have a direct dissolving effect on calcium oxalate or calcium phosphate stones. In fact, excessive alkalinization of the urine in some cases can even lead to the precipitation of other substances, potentially exacerbating the stone-forming conditions.
Another crucial factor is the size and location of the kidney stone. Small stones, especially those less than 5 millimeters in diameter, have a higher chance of passing through the urinary tract on their own. In such cases, sodium bicarbonate might assist by optimizing the urine environment to prevent further growth and promote spontaneous passage. But for larger stones, say those over 10 millimeters, the likelihood of complete dissolution by sodium bicarbonate alone is extremely low. These larger stones often require more invasive procedures like lithotripsy (using shock waves to break the stones into smaller pieces) or surgical removal.
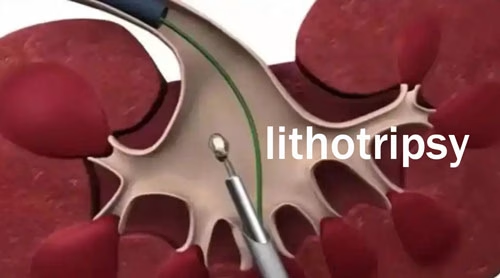
The location of the stone within the kidney or urinary tract also matters. If a stone is lodged in a narrow part of the ureter, it can cause a blockage, leading to severe pain and potential damage to the kidney if not promptly resolved. Sodium bicarbonate, in this situation, would not be sufficient to address the immediate problem of the blockage, even if it could theoretically work on the stone’s composition over time.
Research Findings and Medical Perspectives
Medical research has been delving into the efficacy of sodium bicarbonate in treating kidney stones. Some studies have focused on patients with uric acid stones. In a small clinical trial involving 30 patients, those who were administered sodium bicarbonate along with increased fluid intake showed a significant reduction in stone size over a period of six months. The average initial stone size was around 4 millimeters, and after treatment, nearly 60% of the patients had stones that were less than 2 millimeters, with some showing complete dissolution as detected by ultrasound scans.

However, when it comes to calcium-based stones, the results have been less promising. A retrospective analysis of 100 patients with calcium oxalate stones found that the use of sodium bicarbonate alone did not lead to any appreciable change in stone size or composition. In fact, in about 20% of these patients, there was a slight increase in the stone burden, possibly due to the reasons mentioned earlier regarding the potential for secondary precipitation in an alkalinized environment.
Doctors generally view sodium bicarbonate as a part of a comprehensive treatment approach rather than a standalone cure. For patients with a history of uric acid stones and acidic urine, it can be a useful adjunct to other preventive measures like dietary changes. But they caution against relying solely on it for larger or more complex stones. Dr. Emily Roberts, a urologist with over 20 years of experience, states, “Sodium bicarbonate has its place, especially in the early stages of uric acid stone formation or for prevention. But for patients presenting with large, obstructive stones, we need to consider more aggressive interventions such as extracorporeal shock wave lithotripsy or surgical removal. The key is to accurately diagnose the stone type and tailor the treatment accordingly.”

In conclusion, while sodium bicarbonate shows promise in specific scenarios, particularly for uric acid stones, its use must be carefully evaluated and monitored by medical professionals. Understanding the nuances of kidney stone composition and the patient’s overall condition is essential to harness its potential benefits while minimizing any potential drawbacks.
How to Use Sodium Bicarbonate in Kidney Stone Treatment (If Applicable)
If your doctor determines that sodium bicarbonate could be beneficial for your kidney stone condition, it is crucial to follow their precise instructions. The dosage and frequency of sodium bicarbonate intake will vary depending on factors such as the type and severity of the kidney stones, your overall health, and the current pH level of your urine.
Typically, for adults dealing with uric acid stones, a common starting dose might be around 0.5 to 2 grams, taken several times a day. However, this is a general guideline, and your doctor may adjust it based on your specific circumstances. It’s important not to self-medicate or deviate from the prescribed regimen.

Regular check-ups are essential. Your doctor will likely monitor your progress through various means, including urine tests to measure the pH level. Maintaining the urine pH within a specific target range is key. If the pH becomes too alkaline, it could lead to other issues, as mentioned earlier. By closely tracking the pH, the doctor can make timely adjustments to the sodium bicarbonate dosage.
In addition to taking sodium bicarbonate, other lifestyle modifications play a complementary role. Drinking plenty of water is of utmost importance. Adequate hydration helps flush out the kidneys and urinary tract, preventing the concentration of substances that could form stones. You should aim to drink at least 2 to 3 liters of water per day, unless otherwise advised by your doctor.

Dietary adjustments also go hand in hand. For those with uric acid stones, reducing the intake of purine-rich foods like red meat, organ meats, and certain seafood can be beneficial. Incorporating more fruits and vegetables, which tend to have an alkalizing effect on the body, can support the treatment. Limiting salt and sugar intake is also advisable, as excessive amounts can contribute to stone formation.
Overall, the use of sodium bicarbonate in kidney stone treatment should be a coordinated effort between you and your healthcare provider, with a focus on comprehensive care to achieve the best possible outcome.
Conclusion
In conclusion, the role of sodium bicarbonate in dissolving kidney stones is a complex and nuanced one. While its alkaline nature offers potential benefits, particularly for uric acid stones, it is by no means a one-size-fits-all solution. The effectiveness hinges on multiple factors, including the specific composition, size, and location of the kidney stones, as well as the overall health and urinary chemistry of the patient.
It is crucial for patients to resist the temptation of self-medication and to always consult with a healthcare professional before embarking on any treatment regimen involving sodium bicarbonate. Incorrect or unmonitored use could not only fail to address the problem but might even exacerbate it, leading to further complications.
Moreover, prevention remains a cornerstone in the battle against kidney stones. Maintaining a healthy lifestyle, staying hydrated, and adhering to a balanced diet can go a long way in reducing the risk of stone formation. By understanding the intricacies of kidney stone treatment and prevention, individuals can take proactive steps to safeguard their kidney health and overall well-being.
Expand reading:
Can sodium bicarbonate cause high blood pressure.
Can aspirin be mixed with sodium bicarbonate.
Can sodium bicarbonate tablets lower alkaline phosphatase transplant patients.

