I. Introduction
Clay is often present in various industrial processes and natural environments. Their dispersion is of great significance in many fields such as ceramics, construction, and environmental science. To emerge as a crucial agent in promoting the dispersion of clay particles is one of uses of sodium hexametaphosphate. Understanding the mechanism behind this process is essential for optimizing its applications and improving the quality of related products.
II. Structure and Properties of Sodium Hexametaphosphate
It is necessary to know ” what is sodium hexametaphosphate ” before discussing.
A. Chemical Structure
Sodium hexametaphosphate has the chemical formula (NaPO₃)₆. It consists of a ring-like structure composed of phosphate tetrahedra. Each phosphate tetrahedron shares oxygen atoms with adjacent tetrahedra, forming a cyclic structure. This unique structure gives sodium hexametaphosphate certain chemical reactivity and functional groups that play a vital role in interacting with clay particles.
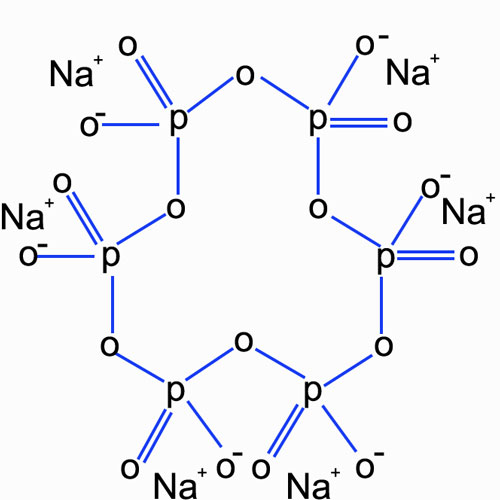
B. Physical and Chemical Properties
It is a white, water-soluble solid. In an aqueous solution, it dissociates into sodium ions and polyphosphate anions. The polyphosphate anions have multiple negative charges distributed along the chain or ring structure. These negative charges are the key to its interaction with clay particles. The solubility of sodium hexametaphosphate in water allows it to be easily introduced into systems containing clay particles.
III. Structure and Characteristics of Clay Particles
A. Chemical Composition and Structure of Clay
Clay minerals are typically composed of silicate sheets. There are mainly two types of sheets: tetrahedral sheets and octahedral sheets. The combination and stacking of these sheets result in different types of clay minerals such as kaolinite, montmorillonite, and illite. For example, montmorillonite has a structure with expandable interlayers between the silicate sheets, which can absorb water and other molecules.
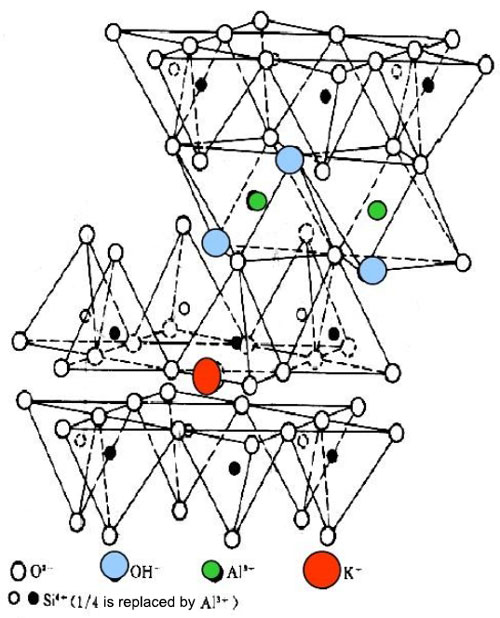
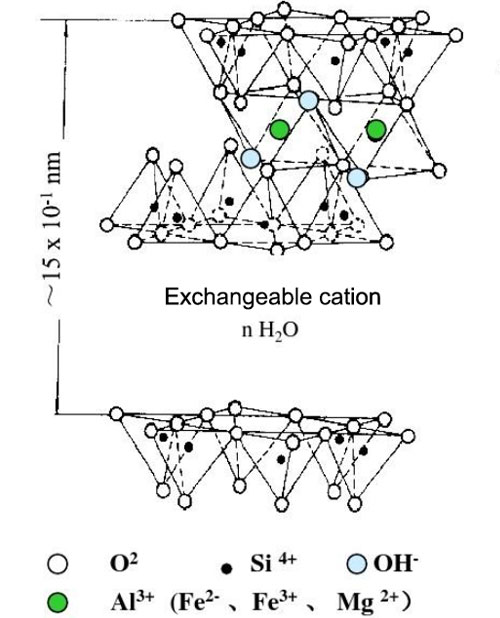
B. Surface Charge and Properties of Clay Particles
Clay particles have a net negative surface charge in most cases. This negative charge is due to the substitution of ions within the crystal structure. For instance, in some clay minerals, aluminum ions may be substituted by magnesium ions in the octahedral sheet, leading to a negative charge imbalance. This negative surface charge makes clay particles prone to interact with positively charged species or other molecules that can neutralize or modify the charge.
IV. Mechanisms of Sodium Hexametaphosphate in Promoting Clay Particle Dispersion
A. Charge Neutralization and Modification
1. When sodium hexametaphosphate is added to a system containing clay particles, the negatively charged polyphosphate anions interact with the positively charged sites on the clay surface. In some cases, there may be edge sites on clay particles that have a positive charge due to broken bonds or other factors. The polyphosphate anions can bind to these sites, reducing the overall net charge difference between adjacent clay particles. This charge neutralization weakens the attractive forces between the clay particles, such as van der Waals forces and electrostatic attractions.
2. Additionally, the polyphosphate anions can also modify the surface charge distribution of the clay particles. They can form a layer of negative charge on the clay surface, which further repels adjacent clay particles. This repulsion helps to overcome the attractive forces and promotes the separation of clay particles. For example, in a clay-water suspension, this charge modification can lead to a more stable suspension with better dispersion of the clay particles.
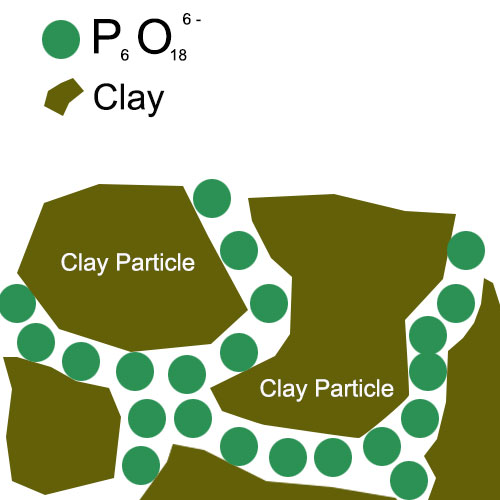
B. Steric Hindrance Effect
1. The polyphosphate anions of sodium hexametaphosphate have a relatively large molecular structure. When they adsorb onto the clay surface, they create a steric hindrance effect. The adsorbed polyphosphate chains extend out from the clay surface, preventing adjacent clay particles from approaching each other closely. This steric effect is especially significant when the clay particles are in a concentrated suspension or in a system with limited space.
2. The steric hindrance provided by the sodium hexametaphosphate molecules also helps to maintain the stability of the dispersed clay particles. It prevents the re-aggregation of the clay particles by physically blocking their contact. In ceramic slurries, for example, this steric effect ensures that the clay particles remain well-dispersed during the shaping process, which is crucial for obtaining high-quality ceramic products with uniform properties.
C. Complexation with Metal Ions
1. Clay particles often contain metal ions either within their structure or adsorbed on the surface. Sodium hexametaphosphate can complex with these metal ions. For instance, calcium and magnesium ions are commonly associated with clay minerals in natural environments. The polyphosphate anions of sodium hexametaphosphate can form stable complexes with these metal ions. This complexation process reduces the influence of metal ions on the aggregation of clay particles.
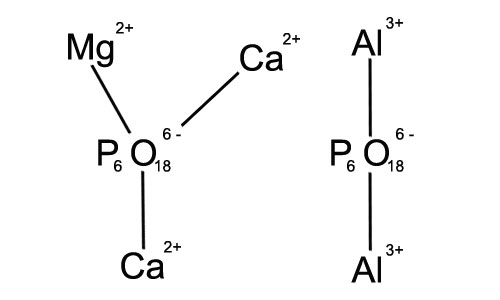
2. Metal ions can act as bridges between clay particles, promoting their aggregation. By complexing with these metal ions, sodium hexametaphosphate disrupts this bridging effect. In the case of soil remediation or in some industrial wastewater treatment processes where clay particles are present along with metal ions, this complexation mechanism helps to disperse the clay particles and improve the overall treatment efficiency.
V. Factors Affecting the Dispersion Effect of Sodium Hexametaphosphate on Clay Particles
A. Concentration of Sodium Hexametaphosphate
1. The concentration of sodium hexametaphosphate in the system has a significant impact on the dispersion of clay particles. At low concentrations, there may not be enough polyphosphate anions to effectively cover the clay surface and provide sufficient charge neutralization or steric hindrance. As the concentration increases, more polyphosphate anions can interact with the clay particles, leading to better dispersion. However, there is an optimal concentration range. Excessive concentrations may cause over-stabilization or other unwanted effects.
2. In laboratory experiments, it has been found that for a particular type of clay suspension, when the concentration of sodium hexametaphosphate is within a certain range, the zeta potential of the clay particles increases significantly, indicating better dispersion. Beyond this range, the zeta potential may not change further or may even show a decrease due to the formation of aggregates caused by excessive anions.
B. pH of the System

1. The pH of the solution containing clay particles and sodium hexametaphosphate affects the dispersion process. At different pH values, the surface charge of the clay particles and the dissociation state of sodium hexametaphosphate change. In an acidic environment, the surface charge of the clay particles may be affected by the presence of hydrogen ions. Some acidic groups on the clay surface may become protonated, changing the charge characteristics. At the same time, the polyphosphate anions of sodium hexametaphosphate may also be affected by the acidic conditions, with possible hydrolysis or changes in their charge distribution.
2. In a basic environment, the clay surface charge may be more stable, but the behavior of sodium hexametaphosphate may also change. The hydrolysis of polyphosphate may occur more readily, which can influence the interaction with the clay particles. Studies have shown that there is an optimal pH range for the dispersion of certain types of clay particles using sodium hexametaphosphate, usually around neutral to slightly basic pH values for many common clay minerals.
C. Temperature of the System
1. Temperature can affect the kinetic energy of the molecules and ions in the system. At higher temperatures, the movement of clay particles, sodium hexametaphosphate molecules, and water molecules becomes more vigorous. This can enhance the interaction between sodium hexametaphosphate and clay particles to some extent. The adsorption rate of polyphosphate anions onto the clay surface may increase with temperature within a certain range.

2. However, high temperatures can also have negative effects. Excessive heat may cause the decomposition of sodium hexametaphosphate or changes in the structure of the clay particles. In industrial applications such as in ceramic production, the temperature during the mixing and shaping processes needs to be carefully controlled to ensure the optimal dispersion effect of the clay particles with the help of sodium hexametaphosphate.
VI. Experimental Methods for Studying the Dispersion of Clay Particles by Sodium Hexametaphosphate
A. Zeta Potential Measurement
1. Zeta potential measurement is a commonly used technique to study the surface charge and stability of clay particles in a suspension. By using a zeta potential analyzer, the electrical potential at the shear plane of the clay particles can be determined. When sodium hexametaphosphate is added to the clay suspension, the change in zeta potential can be monitored. An increase in zeta potential indicates better dispersion as the repulsion between the particles is enhanced.
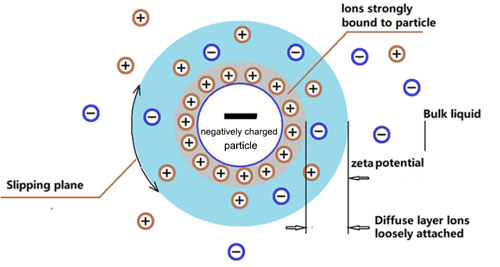
2. In a series of experiments, different concentrations of sodium hexametaphosphate can be added to the clay suspension, and the zeta potential can be measured at different time intervals. This allows us to study the kinetics of the dispersion process and the relationship between the concentration of sodium hexametaphosphate and the zeta potential.
B. Particle Size Analysis
1. Particle size analysis methods such as laser diffraction and dynamic light scattering can be used to determine the size distribution of clay particles in the presence and absence of sodium hexametaphosphate. In a well – dispersed system, the clay particles should have a smaller average particle size and a more uniform size distribution. By comparing the particle size data before and after adding sodium hexametaphosphate, the effectiveness of the dispersion can be evaluated.
2. These methods can also provide information about the stability of the dispersed clay particles over time. If the particle size remains relatively constant during a certain period, it indicates that the dispersion is stable, which is likely due to the action of sodium hexametaphosphate in preventing particle aggregation.
C. Rheological Measurements
1. Rheological measurements can provide insights into the flow behavior of clay-sodium hexametaphosphate suspensions. The viscosity and shear-thinning or – thickening behavior of the suspension can be measured using a rheometer. In a well-dispersed clay suspension with sodium hexametaphosphate, the viscosity may be lower compared to an aggregated system. The shea -thinning behavior may also be more pronounced, indicating that the particles can move more freely under shear stress.
2. By changing parameters such as the concentration of sodium hexametaphosphate and the shear rate, the rheological properties of the suspension can be studied in detail. This helps to understand how the dispersion of clay particles affects the overall rheological behavior of the system, which is important in applications such as in the formulation of ceramic slurries or drilling muds.

VII. Applications of Sodium Hexametaphosphate – Mediated Clay Particle Dispersion
A. Ceramic Industry
1. In the ceramic industry, the dispersion of clay particles is crucial for the quality of ceramic products. Sodium hexametaphosphate is widely used to improve the fluidity of ceramic slurries. By promoting the dispersion of clay particles, it allows for better casting and shaping of ceramic pieces. The well-dispersed clay particles result in a more uniform microstructure in the fired ceramic product, reducing the occurrence of defects such as cracks and voids.
2. During the preparation of ceramic glazes, sodium hexametaphosphate can also help to disperse the clay and other solid components in the glaze formulation. This ensures that the glaze has a smooth and homogeneous texture, which improves the appearance and durability of the glazed ceramic surface.
B. Construction Industry
1. In concrete production, clay particles can sometimes be present as impurities in the aggregate. Sodium hexametaphosphate can be used to disperse these clay particles, preventing them from interfering with the setting and hardening of the concrete. It helps to improve the workability of the concrete mixture by reducing the viscosity caused by the presence of agglomerated clay particles.
2. In the production of building materials such as bricks and tiles, the dispersion of clay particles with sodium hexametaphosphate can enhance the quality of the raw materials. It allows for better compaction and shaping of the materials, resulting in stronger and more durable products.
C. Environmental Applications
1. In wastewater treatment, clay-based adsorbents are sometimes used. Sodium hexametaphosphate can be used to disperse the clay particles in the adsorbent, increasing its surface area and adsorption capacity. This is beneficial for the removal of pollutants such as heavy metals and organic compounds from wastewater.

2. In soil remediation projects, the dispersion of clay particles in the soil can be enhanced by sodium hexametaphosphate. This can improve the mobility of contaminants within the soil, making it easier to remove them through techniques such as soil washing or bioremediation.
There are also many other sodium hexametaphosphate uses as dispersant, but not for the clay.
VIII. Conclusion
Sodium hexametaphosphate plays a crucial role in promoting the dispersion of clay particles through multiple mechanisms including charge neutralization, steric hindrance, and complexation with metal ions. The dispersion effect is affected by factors such as the concentration of sodium hexametaphosphate, the pH and temperature of the system. Understanding these mechanisms and factors is essential for effectively using sodium hexametaphosphate in various industrial and environmental applications related to clay particle dispersion. Experimental methods such as zeta potential measurement, particle size analysis, and rheological measurements provide valuable tools for studying this process. The wide range of applications in the ceramic, construction, and environmental sectors highlights the importance of sodium hexametaphosphate – mediated clay particle dispersion in improving product quality and environmental remediation processes.
Expand reading:

