I. Introduction
In the realm of chemistry and various industries, the ability to separate sodium carbonate (Na2CO3), commonly known as soda ash or washing soda, and sodium bicarbonate (NaHCO3), familiarly called baking soda, holds great significance.

In industry the raw material for sodium bicarbonate is sodium carbonate, while in the household sodium bicarbonate can also be made from sodium bicarbonate (see the article How to Make Sodium Carbonate from Sodium Bicarbonate). Thus, the two appear to be impurities of each other.
In this paper, we will present an approach that takes advantage of their different solubility in water to achieve the separation.
II. Solubility Difference Method
A. Leveraging Solubility Variations
Sodium carbonate and sodium bicarbonate display distinct solubility behaviors in water, especially with respect to temperature changes.
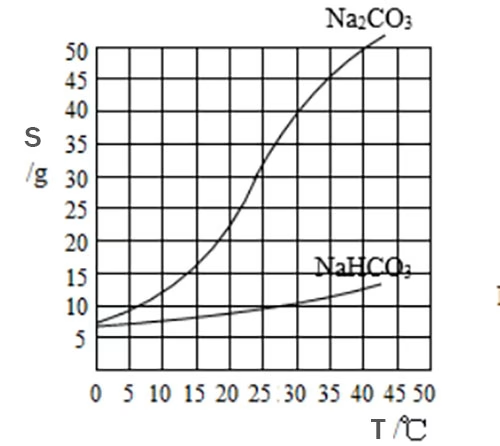
Sodium carbonate is highly soluble in water, and its solubility exhibits a positive correlation with temperature. As the temperature rises, more sodium carbonate can dissolve in the water. For example, at 20 °C, approximately 21.5 g of sodium carbonate can dissolve in 100 g of water. When the temperature is increased to 100 °C, the solubility shoots up to around 45.5 g per 100 g of water.

On the other hand, sodium bicarbonate, while also soluble in water, has a relatively lower solubility that changes less dramatically with temperature. At 0 °C, its solubility is about 6.9 g per 100 g of water. At 20 °C, it increases to around 9.6 g per 100 g of water, and at 60 °C, it reaches approximately 16.5 g per 100 g of water. This difference in solubility trends provides a crucial foundation for separation.
By carefully adjusting the temperature of a saturated or near-saturated solution containing both compounds, it is possible to selectively precipitate one compound while keeping the other in solution, thereby achieving separation.
B. Step-by-step Separation Process
Preparation of the Solution:
Begin by weighing out a known amount of the mixture containing sodium carbonate and sodium bicarbonate.
Place this mixture into a beaker and add distilled water gradually while stirring continuously. The amount of water added should be such that the solution is either saturated or near-saturated at the starting temperature. This temperature is typically chosen to be around room temperature (20 – 25 °C), but it can be adjusted depending on the specific composition of the mixture and the desired separation outcome.
Heating the Solution:
Slowly heat the beaker containing the solution using a hot plate or a water bath.
As the temperature rises, monitor the solubility changes. The goal is to reach a temperature where the solubility difference between the two compounds is maximized.

For instance, a temperature range of 50 – 60 °C is often suitable. As the solution heats up, sodium carbonate, with its higher solubility at elevated temperatures, will remain in solution, while sodium bicarbonate, which has a relatively lower solubility increase, may start to approach its saturation point.
Precipitation and Separation:
Once the target temperature is reached, continue to stir the solution gently for a few minutes to ensure equilibrium.
Then, slowly cool the solution. As the temperature decreases, sodium bicarbonate, which is now closer to its solubility limit, will start to precipitate out of the solution. This precipitation can be observed as fine crystals forming in the beaker.

To aid in the separation, a filter funnel lined with filter paper can be used. Pour the solution through the funnel, and the precipitated sodium bicarbonate crystals will be retained on the filter paper. The filtrate, which now contains predominantly sodium carbonate in solution, can be further processed. This might involve evaporating the water to obtain solid sodium carbonate.
C. Considerations and Challenges
Impurity Removal:
During the separation process, it is essential to consider the presence of impurities.
The starting mixture may contain other substances that can affect the solubility and separation.
For example, if there are traces of salts or other ionic compounds, they could potentially co-precipitate with either sodium carbonate or sodium bicarbonate, leading to impure products.

To address this, a preliminary purification step might be necessary. This could involve techniques such as washing the precipitate with cold distilled water to remove any surface-adhered impurities or using ion exchange resins to selectively remove unwanted ions from the solution before the separation process.
Solution Saturation:
Maintaining the appropriate level of saturation is crucial. If the initial solution is not saturated enough, it may not be possible to achieve effective separation as the solubility differences will not be fully exploited.
Conversely, if the solution is over-saturated, there is a risk of rapid and uncontrolled precipitation, which can lead to the formation of large clumps that trap other compounds, again resulting in impure products.
Careful measurement of the amounts of solute and solvent, as well as precise control of temperature changes, is required to strike the right balance and ensure optimal separation.
Additionally, the rate of cooling can also impact the quality of the precipitate. A slow, controlled cooling rate generally promotes the formation of small, uniform crystals, which are easier to separate and purify compared to large, irregularly formed ones.
III. Conclusion
In summary, we have explored a technique for separating sodium carbonate and sodium bicarbonate. The solubility difference method capitalizes on the varying solubilities of sodium carbonate and sodium bicarbonate in water with temperature changes. Through careful manipulation of temperature and saturation levels, selective precipitation of one compound can be achieved, followed by filtration and further processing.
Looking ahead, there is potential for further advancements in the separation of sodium carbonate and sodium bicarbonate.
Emerging research in materials science and chemical engineering may lead to the development of more efficient and selective separation membranes. These membranes could potentially separate the two compounds based on differences in molecular size, charge, or other properties, offering a continuous and energy-efficient separation process.
Additionally, advancements in process intensification techniques could combine multiple separation steps into a single unit operation, reducing equipment footprint and energy consumption. For example, coupling thermal decomposition with in situ separation of the evolved gases and recycling of heat could enhance the overall efficiency of the process.
As industries continue to demand higher purity chemicals and more sustainable production methods, further research and innovation in this area will be crucial to meet these evolving needs.
Whether it is in the food, pharmaceutical, or industrial sectors, improved separation techniques for sodium carbonate and sodium bicarbonate will contribute to enhanced product quality, reduced waste, and increased competitiveness.
Expand reading:

