I.Introduction
Sodium tripolyphosphate and trisodium phosphate are two important compounds that play significant roles in various industries. Understanding the differences and similarities between these two substances is crucial for many applications.
Sodium tripolyphosphate (also known as STPP), is a widely used compound. It is a sodium salt of triphosphoric acid and is commonly found in many products. STPP is often used as a builder in soaps and detergents. It is also used in food additives, paper production, paint manufacturing, and ore flotation. Additionally, it serves as a stabilizer for hydrogen peroxide solutions.
Trisodium phosphate(TSP), on the other hand, is also an important compound. It is known as sodium phosphate or phosphoric acid trisodium salt. Trisodium phosphate is a white crystalline solid that is soluble in water. It is used in various applications such as cleaning agents, water treatment, and food processing.
II.History and Development
A. Sodium Tripolyphosphate
The history of sodium tripolyphosphate dates back to the early days of the phosphate industry. Discovered by researchers, it has seen significant development over the years. Sodium tripolyphosphate was first used in various applications in the early 20th century. In the westen countries, the phosphate industry began in the early 1900s. The United States started producing polyphosphates in 1940 and began mass production of sodium tripolyphosphate in 1947. As the use of synthetic detergents increased, the production of STPP grew rapidly. In Japan, the production of sodium tripolyphosphate also grew along with the increase in synthetic detergents. In the United Kingdom, wet-process production of sodium tripolyphosphate began in 1950.

In the 1990s, the usage of sodium tripolyphosphate increased rapidly, and production scale continued to expand, becoming one of the top ten major phosphate products. In the 21st century, the sodium tripolyphosphate industry is further developing in the direction of refinement, systematization, and quality improvement.
B. Trisodium Phosphate
Trisodium phosphate has a long history as well. Its origins can be traced back to different industries where it gradually gained importance. Trisodium phosphate is known as sodium phosphate or phosphoric acid trisodium salt. It is a white crystalline solid that is soluble in water. In the early days, it was used in various applications such as cleaning agents and water treatment. As industries developed, its usage expanded to food processing and other fields.

Significant events or developments related to trisodium phosphate’s production and application include its role in different industries due to its properties( click to understand trisodium phosphate properties). In cleaning agents, it acts as an effective cleaner due to its properties. In water treatment, it helps in removing impurities and softening water. In food processing, it is used for various purposes such as adjusting pH levels and as a food additive in some cases. Its importance lies in its versatility and effectiveness in different applications.
III.Contrasts
Sodium tripolyphosphate (STPP) and trisodium phosphate have both similarities and differences that are crucial for their applications in various industries.
A.Similarities:
- Both are important compounds with significant roles in different industries.
- They are both soluble in water and have certain chemical reactivity.
- Both can be used in applications related to cleaning, water treatment, and food processing.
B.Differences:
- Chemical Formula: STPP has a chemical formula of Na5P3O10, while trisodium phosphate is Na3PO4.
- Chemical Structure: STPP is an amorphous linear polyphosphate that terminates at both ends with Na2PO4 and forms regular tetrahedrons. Trisodium phosphate is a white crystalline solid with a simpler molecular structure.
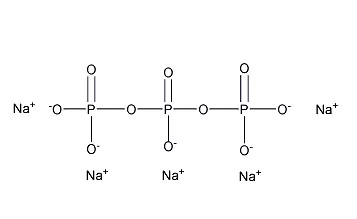
- Physical Properties: STPP has two types of crystals with different stability temperatures. It has a relatively high solubility at room temperature and a specific melting point. Trisodium phosphate is also a white crystalline solid but has different solubility and melting characteristics.
- Preparation Methods: STPP can be prepared through hot process with phosphoric acid and soda ash or caustic soda, wet process, and white carbon black co-production method. Trisodium phosphate is produced by reacting phosphoric acid with sodium hydroxide.
- Uses and Applications: STPP is widely used as a builder in detergents, food additive, paper making, mining, and hydrogen peroxide stabilizer. Trisodium phosphate is used as a cleaner, water softener, detergent, corrosion inhibitor, pH adjuster, and in food processing.
- Safety and Toxicity: Both have relatively low toxicity, but excessive intake can cause health risks. STPP may lead to nausea, vomiting, diarrhea, and low calcium-induced tetany. Trisodium phosphate is a strong alkali and can cause skin and eye irritation.
C.Importance in Various Industries:
- In the detergent industry, STPP acts as a builder to enhance cleaning power, while trisodium phosphate is also used as a detergent.
- In food processing, STPP is a food additive for stabilizing, emulsifying, and moisture retention. Trisodium phosphate is used for pH adjustment.
- In water treatment, both can help remove impurities and soften water.
D.Potential for Future Applications:
As industries continue to develop, there is potential for further research and innovation in the use of STPP and trisodium phosphate.

For example:
- In the field of environmental protection, more sustainable preparation methods and applications could be explored.
- In the food industry, there may be opportunities to develop new food additives with improved properties using these compounds.
- In water treatment, they could play an important role in addressing water quality issues and meeting the growing demand for clean water.
IV. Conclusion
In conclusion, sodium tripolyphosphate and trisodium phosphate are valuable compounds with unique properties and applications. Understanding their differences and similarities is essential for industries to make informed decisions and optimize their processes. Their importance in various industries and potential for future applications make them key substances in the chemical industry.
Other contrasts:
Sodium Hexametaphosphate vs sodium tripolyphosphate.
Trisodium Phosphate vs Sugar Soap: Are They the Same?

