I. Introduction
Disodium phosphate is a widely recognized compound with Cas No. 7558-79-4. It has significant applications in both food processing and chemical experiments.

It is commonly found in the form of powders or crystals. It has a defined molecular weight of around 141.96 g/mol. Its physical and chemical properties make it a valuable compound in multiple industries. Whether it’s in the production of processed foods or in the conduction of scientific research, disodium phosphate continues to be an important compound with diverse applications.
II. Chemical Formula and Properties
A. Formula and Molecular Weight
Disodium phosphate has the chemical formula Na2HPO4. The molecular weight of disodium phosphate is approximately 141.96 g/mol as mentioned earlier. This molecular weight is calculated based on the atomic masses of hydrogen (H), sodium (Na), oxygen (O), and phosphorus (P).
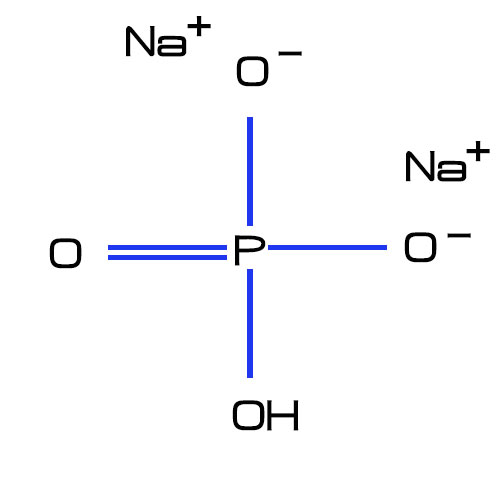
B. Physical Properties
Disodium phosphate ( Na2HPO4) is colorless or white crystal, containing 1~12 molecules of water of crystallization.
In the air, it can quickly weather to disodium phosphate heptahydrate.
When heated to 100°C, disodium phosphate loses all its water of crystallization and becomes anhydrous(disodium phosphate). When heated to 250°C, disodium phosphate will decompose into sodium pyrophosphate.
It is easily soluble in water, almost completely decomposed into disodium hydrogen phosphate and sodium hydroxide in water, and its aqueous solution is strongly alkaline, insoluble in ethanol, carbon disulfide.

Disodium phosphate is soluble in water. According to the search results, it has a solubility of H2O: 0.5 M concentration.
The compound has a melting point of 243 – 245 °C. It has no specific data available for boiling point.
C. Chemical Properties
In terms of chemical properties, disodium phosphate undergoes hydrolysis. The dissociation reactions result in the release of sodium ions and phosphate ions.
In acidified ammonium molybdate solution, adding disodium hydrogenphosphate solution while it is still hot produces a yellow crystalline precipitate with the molecular formula, Its molecular formula is 12-molybdenum ammonium phosphate, chemical formula (NH4)3[P(Mo12O40)]-6H2O
The presence of specific ions can be determined by observing the yellow crystalline precipitate produced.

The process by which ions ionized in aqueous solution interact with hydrogen and hydroxide ions in water to produce a weak electrolyte. The ionic equation for the hydrolysis of disodium phosphate is as follows:
- Primary hydrolysis: PO43-+H2O⇌HPO42-+OH–
- Secondary hydrolysis: HPO42-+H2O ⇌ H2PO4-+OH–
- Tertiary hydrolysis: H2PO4–+H2O ⇌ H3PO4+OH –
IV. Related question about Disodium phosphate
A. Is disodium phosphate an acid or base?
Disodium hydrogen phosphate is readily soluble in water, forming an alkaline solution, due to its chemical structure and the nature of the hydrolysis reaction.

B. Is disodium phosphate an ionic or covalent bond?
Sodium ions (Na+) and hydrogen phosphate ions (HPO4-) in disodium phosphate are attracted to each other through electrostatic interactions to form stable compounds, which is a typical ionic bonding.
C. Is disodium phosphate an organic or inorganic compound?
Disodium hydrogen phosphateis an inorganic compound.
D. Can Mg react with disodium hydrogen phosphate?
In aqueous solution, disodium hydrogen phosphate reacts with magnesium ions(Mg2+) in water. After the water treatment agent containing disodium hydrogen phosphate is added to the water, the phosphate ions therein will ion exchange with the calcium (Ca2+)and magnesium ions (Mg2+)in the water to generate insoluble calcium phosphate and magnesium phosphate. This is the principle of water softening with disodium hydrogen phosphate.
V. Conclusion
Disodium phosphate is a compound of significant importance in multiple fields. In food processing, it acts as a buffering agent, ensuring the stability and quality of food products. Its ability to maintain a stable pH level is crucial for the proper functioning of various food formulations.
In chemical experiments, disodium phosphate plays a vital role. Its solubility in water makes it suitable for a wide range of chemical processes. It is also useful in chemical analysis due to its characteristic reactions, such as the formation of a yellow precipitate when reacting with molybdate.
The classification of disodium phosphate as a simple phosphate.
In conclusion, disodium phosphate is a valuable compound with diverse applications and significance in different fields. Its importance lies in its ability to contribute to the stability and quality of food products, as well as its role in chemical experiments and analysis. As our understanding of chemistry continues to advance, the applications and significance of disodium phosphate are likely to expand further.

