I. Introduction of Sodium Pyrophosphate
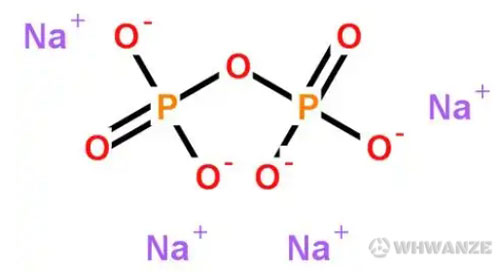
Sodium pyrophosphate is an inorganic compound with cation sodium and anion pyrophosphate, and its chemical formula Na4P2O7 . It is also known as tetrasodium pyrophosphate.
It is a white, crystalline powder that is highly soluble in water. It is widely used in various industries due to its unique properties. The compound is formed by the combination of four sodium ions (Na+) and one pyrophosphate anion (P2O74-).
English aliases for sodium pyrophosphate include:
Tetrasodium pyrophosphate.
Sodium pyrophosphate, anhydrous.
SPP.
TSPP.
Tetrasodium pyrophosphate decahydrate.
TSPP decahydrate.
Sodium pyrophosphate decahydrate.
In the chemical industry, sodium pyrophosphate plays an important role as a chelating agent, sequestering metal ions and preventing their precipitation. It is also used as a buffering agent to maintain a stable pH in various chemical processes.
II. Physical and Chemical Properties
A. Appearance and Solubility
Sodium pyrophosphate appears as a white powder or crystal. It is highly soluble in water, which makes it useful in various applications where it needs to be easily dispersed. However, it is insoluble in alcohol. This solubility characteristic allows it to be easily incorporated into aqueous solutions for different industrial processes and in the food industry.

B. Stability and Reactivity
1. Hydroysis
Sodium pyrophosphate is stable below 70°C in water. However, when sodium pyrophosphate is heated to the boiling point in water, a hydrolysis reaction occurs to produce disodium hydrogen phosphate (Na2HPO4) and sodium phosphate (Na3PO3) with the following chemical equation
This means that in high-temperature conditions, it breaks down into simpler compounds.
2. It reacts with metal ions to form complexes.
For example, it can sequester metal ions, preventing their precipitation and playing an important role as a chelating agent in the chemical industry. These complexes can have different properties and applications depending on the metal ions involved.
C. pH Characteristics
Sodium pyrophosphate has an alkaline aqueous solution with a pH value of 10.0 – 10.2. As a polymerized phosphate, it has common properties such as the ability to act as a buffer. This means it can help maintain a relatively stable pH in solutions. In various chemical processes and in the food industry, this property is valuable as it can help control the acidity or alkalinity of a system. For example, in food products, it can help maintain a stable pH to ensure the quality and safety of the food. For detailed explanation, please read “is sodium pyrophosphate an acid or base“.
III. Preparation Methods
Sodium Pyrophosphate Preparation Methods
Sodium pyrophosphate can be prepared through one-step and two-step methods.
The one-step method typically involves the reaction of sodium carbonate or sodium hydroxide with phosphoric acid or pyrophosphoric acid under specific conditions. For example, heating a mixture of sodium carbonate and phosphoric acid to a certain temperature can lead to the formation of sodium pyrophosphate. This method is relatively straightforward but requires careful control of reaction conditions to ensure high purity and yield.
The two-step method usually consists of two separate reactions. In the first step, a precursor compound is formed. For instance, sodium dihydrogen phosphate can be prepared by reacting sodium carbonate with phosphoric acid. In the second step, the precursor is heated to a higher temperature to form sodium pyrophosphate. This method allows for better control over the reaction process and can result in a more pure product.
The choice of preparation method depends on various factors such as the availability of raw materials, desired purity, and cost considerations. In industrial production, different methods may be employed based on specific requirements.
For the food grade sodium pyrophosphate, it could be prepared by both one-step method and two-step method. They are using the food grade material. The difference is one-step method may difficult to control the quality.
In conclusion, both the one-step and two-step methods have their own advantages and are used in the preparation of sodium pyrophosphate depending on the specific circumstances.
IV. Conclusion
Sodium pyrophosphate is a valuable compound with wide-ranging applications. While it offers many benefits, it is essential to be aware of potential risks associated with excessive intake and use it in moderation. Continued research and monitoring are needed to better understand its long-term effects and ensure its safe use in different fields.
Expand reading:
Sodium pyrophosphate uses in food.

