I. Introduction to Disodium Hydrogen Phosphate Heptahydrate
A. Chemical Identity
Disodium hydrogen phosphate heptahydrate ( Cas No. 7782-85-6) has a specific chemical structure. The formula Na2HPO4·7H2O indicates that it consists of two sodium atoms (Na), one hydrogen atom (H), one phosphorus atom (P), and four oxygen atoms from the phosphate group (PO4), along with seven molecules of water (H2O). This compound forms white crystalline powder.
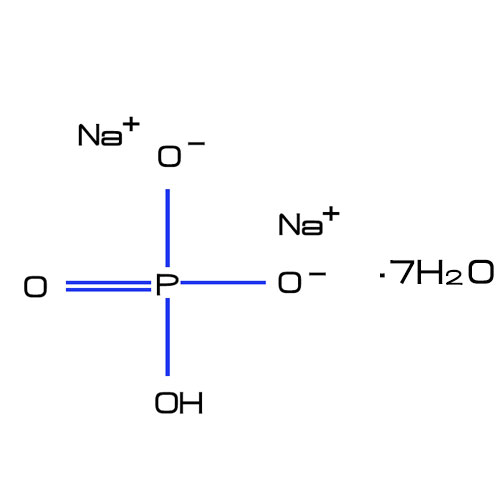
So, disodium hydrogen phosphate heptahydrate molecular weight is 268.066 .
B. Nomenclature
As mentioned, it is known by several names. “Sodium phosphate dibasic heptahydrate” emphasizes its nature as a salt with two sodium atoms and the presence of seven water molecules. “Disodium hydrogen phosphate heptahydrate” also clearly describes its composition with two sodium atoms and a hydrogen phosphate group along with seven water molecules. This compound is widely recognized in the scientific and industrial communities by these various names.
II. Physical Properties
A. Appearance
Disodium hydrogen phosphate heptahydrate is a white crystalline powder. This characteristic appearance makes it easily distinguishable. The white color is pure and consistent, giving it a clean and professional look. The crystalline structure implies a certain degree of order and regularity in the arrangement of atoms and molecules.

B. Solubility
This compound has a specific solubility profile. It is soluble in water. This means that when placed in water, it dissociates into its constituent ions, namely sodium ions (Na+), hydrogen phosphate ions (HPO42-), and water molecules released from the heptahydrate structure. The solubility in water makes it useful in various applications where it needs to be in a liquid form for ease of handling and use. On the other hand, it is insoluble in ethanol. This property is important as it dictates the choice of solvents for different processes.
For example, in applications where ethanol is used as a solvent, disodium hydrogen phosphate heptahydrate would not dissolve and might not be suitable. According to available data, the solubility in water is a key characteristic that influences its usage in fields such as chemistry, biology, and industry. For instance, in the preparation of certain solutions for laboratory experiments or in industrial processes where a water-soluble compound is required, disodium hydrogen phosphate heptahydrate can be a valuable choice.
III. Uses and Applications
A. In Research
Disodium hydrogen phosphate heptahydrate is widely used in scientific research. As a buffer, it helps maintain a stable pH in solutions, which is crucial for many biochemical and chemical reactions. For example, in biological research, it is used in the preparation of Leptospira Medium Base. In analytical chemistry, it can be used to determine potassium levels. Its stability and known chemical properties make it a reliable reagent in various research settings.

B. In Industry
Disodium hydrogen phosphate heptahydrate is the raw material for the production of sodium hexametaphosphate and condensed acid salt.
In the electroplating process, disodium hydrogen phosphate heptahydrate as one of the components of the plating solution, can help to improve the effect of electroplating, improve the quality of plating layer and uniformity.

In the process of leather tanning, disodium hydrogen phosphate heptahydrate can help the tanning agent of leather to better penetrate and improve the quality and softness of the leather. In the leather tanning process, disodium phosphate heptahydrate can help the leather tanning agent to penetrate better, and improve the quality and softness of leather
IV. Safety Considerations
A. Risk Warnings
Disodium hydrogen phosphate heptahydrate can pose certain risks if not handled properly. It may cause irritation to the eyes, respiratory system, and skin. Direct contact with the eyes can lead to discomfort, redness, and in severe cases, damage to the cornea. Inhalation of dust or fumes from the compound may irritate the respiratory tract, causing coughing, wheezing, and shortness of breath. Skin contact can result in dryness, redness, and itching.
B. Precautions
When handling disodium hydrogen phosphate heptahydrate, it is essential to take proper precautions. Wear appropriate personal protective equipment such as safety glasses, gloves, and a lab coat. Avoid direct contact with the skin and eyes. In case of accidental contact, immediately flush the affected area with plenty of water for at least 15 minutes. If irritation persists, seek medical attention. When working in an area where the compound is present, ensure proper ventilation to prevent the buildup of dust or fumes. Store the compound in a cool, dry place away from incompatible substances. Follow all safety guidelines and instructions provided by the manufacturer to minimize the risks associated with handling disodium hydrogen phosphate heptahydrate.
V. Conclusion
The Disodium hydrogen phosphate heptahydrate is one of the hydrates of disodium phosphate. So, its formula is not the same as disodium phosphate formula. Furthermore, the property and its application are different with the disodium phosphate uses. So, it should not to mix it with the disodium phosphate when you try to buy it from wholesalers.

