I. Let’s start with the conclusion
Sodium fluoride, with the chemical formula NaF, is an inorganic ionic compound.
It is the only hygroscopic salt among alkali metal fluorides and may form lumps upon long-term storage.
II. Understanding ionic and covalent bonding
A. Definition and Characteristics
Ionic bonding occurs when electrons are transferred from one atom to another, resulting in the formation of positive and negative ions. These oppositely charged ions are held together by electrostatic forces. Ionic compounds generally have high melting and boiling points, are hard and brittle, and conduct electricity when molten or in aqueous solution.
Covalent bonding, on the other hand, involves the sharing of electrons between atoms. Covalent compounds often have lower melting and boiling points compared to ionic compounds. They can be in solid, liquid, or gaseous states at room temperature and generally do not conduct electricity.
B. Factors Influencing Bonding
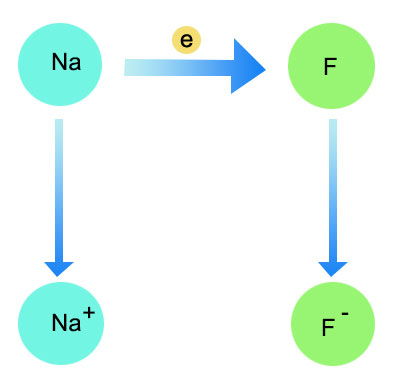
The nature of the elements involved is a major determinant of bonding type. When a metal with low electronegativity combines with a non-metal with high electronegativity, ionic bonding is likely to occur. For example, sodium (a metal) and fluorine (a non-metal) form sodium fluoride through ionic bonding. In this case, sodium readily loses an electron to form a positive ion, while fluorine gains an electron to form a negative ion.
The difference in electronegativity between two atoms is also crucial. A large electronegativity difference typically leads to ionic bonding. In contrast, when the electronegativity values of two atoms are similar or close, covalent bonding is more likely. Elements with similar electronegativities share electrons to achieve a stable electron configuration.
For instance, in compounds formed between two non-metals like carbon and oxygen, covalent bonding occurs as both elements have comparable electronegativities.
Another factor is the size of the atoms. Smaller atoms with high electronegativity tend to form covalent bonds more readily. Larger atoms with lower electronegativity are more likely to form ionic bonds. However, these factors are not absolute and multiple factors need to be considered when determining the bonding type of a compound.
III. Analysis of Sodium Fluoride’s Bonding
A. Molecular Structure
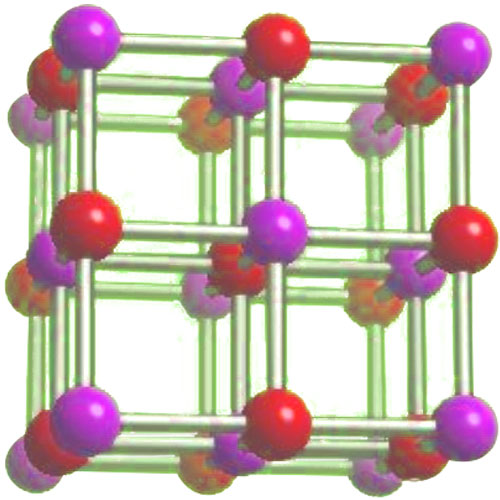
Sodium fluoride consists of sodium cations (Na⁺) and fluoride anions (F⁻). In the crystal structure of sodium fluoride, each sodium ion is surrounded by six fluoride ions, forming a regular octahedral arrangement. This structure is characteristic of ionic compounds. The strong electrostatic attraction between the oppositely charged ions holds the crystal lattice together. This molecular structure clearly indicates that sodium fluoride has ionic bonding.
B. Properties Supporting Ionic Bonding
The solubility of sodium fluoride in water is a property that supports its ionic nature. Ionic compounds tend to dissolve in polar solvents like water because the polar water molecules can surround and separate the ions, forming a solution. Sodium fluoride dissolves in water due to the attraction between the positive end of water molecules (hydrogen atoms) and the fluoride anions, and the negative end of water molecules (oxygen atom) and the sodium cations.
The high melting point of sodium fluoride, around 993 °C, is also indicative of ionic bonding. Ionic compounds have high melting points because a significant amount of energy is required to break the strong electrostatic forces between the ions.
In terms of electrical conductivity, sodium fluoride conducts electricity when molten or in aqueous solution. In the molten state or in solution, the ions are free to move and carry an electric current. This is a characteristic feature of ionic compounds. For example, when sodium fluoride is melted, the sodium ions and fluoride ions can move freely, allowing the flow of electric charge.
IV. Conclusion
Sodium fluoride is clearly an ionic compound as evidenced by its various bonding characteristics and properties. The transfer of an electron from sodium to fluorine results in the formation of sodium cations and fluoride anions, which are held together by strong electrostatic forces characteristic of ionic bonding.
The molecular structure of sodium fluoride, with sodium ions surrounded by fluoride ions in an octahedral arrangement, further confirms its ionic nature. This regular crystal lattice structure is maintained by the strong attractions between the oppositely charged ions.
The properties of sodium fluoride also support its classification as an ionic compound. Its solubility in water is due to the interaction between the polar water molecules and the ions. The high melting point of around 993 °C indicates that a significant amount of energy is required to break the strong ionic bonds. Additionally, its ability to conduct electricity when molten or in aqueous solution is a hallmark of ionic compounds, as the mobile ions can carry an electric current. In conclusion, based on its molecular structure and physical and chemical properties, sodium fluoride is undoubtedly an ionic compound. And this makes it “what is sodium fluoride“.

