I. Introduction
We know that both sodium tripolyphosphate(STPP)and sodium pyrophosphate(SPP) can be applied in the field of water treatment. They are both a kind of phosphate. So as water treatment aids, what are the similarities and differences between them?
This article will list their effects, so that you have a clear perception.

II. Effects of sodium tripolyphosphate in water treatment:
A. Water softening:
Sodium tripolyphosphate has strong chelating ability for hardness ions in water (such as calcium and magnesium ions), through chelation, these ions can combine with sodium tripolyphosphate to form soluble chelates, thus reducing the hardness of water. This not only helps to reduce the generation of scale, but also protects pipes and equipment from scale erosion and extends their service life.
B. Water purification:
Sodium tripolyphosphate can combine with other metal ions in water (such as copper and ferrous ions) to form insoluble precipitates, thus removing the adverse effects of these ions on water quality. In addition, it enhances the bleaching effect, removes odors and other foul tastes caused by metal ions, and improves the overall quality of water.
C. Improvement of washing effect:
In the field of water treatment, especially in the process of industrial washing and cleaning, sodium tripolyphosphate, as one of the main auxiliaries of synthetic detergents, can significantly improve the washing effect. By chelating the hardness ions in the water, it prevents these ions from combining with the active ingredients in the detergent to form precipitation, thus maintaining the activity of the detergent and improving the decontamination ability. At the same time, sodium tripolyphosphate disperses suspended particles and dirt in the detergent, making it easier for them to be washed away by the water.

D. Protecting equipment:
In water treatment systems, equipment is susceptible to erosion and wear and tear from impurities in the water. The use of sodium tripolyphosphate reduces the hardness of the water and minimizes scale formation, thereby protecting pipes, pumps and other equipment from scale. In addition, it reduces scaling and clogging of equipment and improves its operational efficiency and stability. For example, in water purifiers, sodium tripolyphosphate is often used as a scale inhibitor to stop the crystallization and precipitation of calcium and magnesium ions, preventing them from adhering to RO membrane cartridges, protecting the cartridges and prolonging their service life. The scale inhibitor will decompose and most of the components are discharged with the wastewater, but the real concentration in drinking water is very low, basically can be controlled within the safe dose.
E. Environmental protection:
Although excessive use of sodium tripolyphosphate in the environment may lead to problems such as eutrophication of water bodies, it can be used as an environmental protection agent under appropriate usage. For example, in the process of wastewater treatment, sodium tripolyphosphate can be used in conjunction with sodium hexametaphosphate to remove heavy metal ions and other hazardous substances from wastewater and reduce pollution to the environment.
F. Application:
Sodium tripolyphosphate is widely used in household cleaning, agriculture, plating, sewage treatment and other fields. It is suitable for cleaning utensils, clothes, floors, walls and other surfaces, effectively removing various stains.
III. Effects of sodium pyrophosphate in water treatment:
A. Water softening:
sodium pyrophosphate is able to complex with these ions in water to generate stable complexes, thus removing these ions from water. This softening effect is important for preventing the formation of scale and protecting water treatment equipment and pipelines.

B. Scale inhibition:
sodium pyrophosphate can combine with calcium, zinc, magnesium, iron and other ions in the water to generate stable complexes, thus reducing the concentration of these ions and reducing the formation of calcium carbonate and other scale. In addition, sodium pyrophosphate can be adsorbed on the active point of crystals to change the crystal structure and inhibit the growth of calcium carbonate scale crystals. This makes sodium pyrophosphate widely used in oilfield reinjection wastewater treatment systems, boilers, circulating water, cooling towers and other fields.
C. Corrosion inhibition:
Sodium pyrophosphate can complex metal ions to form a protective film, isolating the metal from direct contact with water and preventing the metal from corroding. This protective film can protect metal pipes and equipment from corrosion.

D. Adjustment of water quality:
sodium pyrophosphate can also be used to adjust the pH value of water, so that it stays within the appropriate range. This is very important for the stability of water quality and the smooth running of subsequent treatment processes.
E. Environmental benefits:
The application of sodium pyrophosphate in water treatment has environmental benefits. It can safely and effectively remove harmful substances in water, while protecting water treatment equipment and pipelines from corrosion and scaling, and will not pollute the environment.
F. Applications
Remove sediment, rust and other stains attached to the metal surface; remove heavy metal elements such as iron and manganese in groundwater; it can be used as a phosphatizing agent for the production of dyes and other chemical products.
G. Precautions for use:
When using sodium pyrophosphate for water treatment, you need to pay attention to the following points:
Sodium pyrophosphate is easily hydrolyzed to produce orthophosphoric acid, which may react with calcium ions to produce insoluble calcium phosphate. Therefore, it is necessary to control the dosage and proportion to avoid excessive calcium phosphate precipitation.
The temperature of use should be lower than 80°C to avoid accelerated hydrolysis to affect the effect1.
It is more effective when used under weakly acidic (pH=6-7) water conditions, and exceeding this value may cause localized corrosion or formation of calcium phosphate precipitation.
IV. Conclusion
Both sodium tripolyphosphate and sodium pyrophosphate have very important roles in water treatment, however, there are differences between the two in terms of specific use.
A. Scale inhibitor for water treatment equipment:
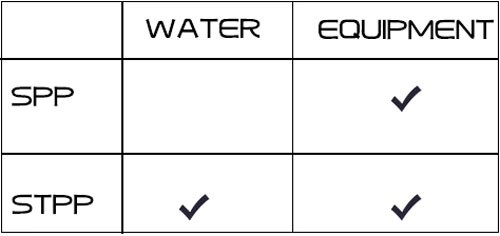
both sodium tripolyphosphate and sodium pyrophosphate can soften water, and used as scale inhibitor to protect the pipeline and equipment. The difference is that sodium tripolyphosphate forms chelates with metal ions, while sodium pyrophosphate forms complexes with metal ions.
B. Sewage treatment:
because sodium pyrophosphate has stronger corrosion inhibition properties, can passivate the metal surface, and strong stability for hard surface cleaning, such as metal surface treatment, pipeline cleaning, industrial equipment cleaning and other occasions, so the main role of sodium pyrophosphate in sewage treatment is in the cleaning and maintenance of sewage treatment equipment. Sodium tripolyphosphate is more widely used in the application, not only can be used for water treatment equipment scale inhibition, but also can be directly used to regulate the pH value of sewage and inhibit the growth of harmful microorganisms, to help maintain the stability of water quality!
C. Detergents.
Sodium tripolyphosphate ensures that detergent actives work effectively by chelating metal ions in water. Sodium pyrophosphate itself has excellent bleaching and dispersing functions and can be directly involved in decontamination.
D. Adverse effects on human body and the environment:
compared with the two, sodium tripolyphosphate is less toxic and less irritating to human body, with high safety.
The above is the difference between the two in the field of water treatment, and the products used here are their industrial grade products. Their food grade products, with higher purity, are mainly used as food additives. Our company supplies food grade sodium tripolyphosphate and sodium phyrophosphate, which are more expensive than industrial grade products. The raw materials used are all food-grade materials, making the products safer and more hygienic.
Related contracts:
Sodium tripolyphosphate vs trisodium phosphate
Related articles:

