Sodium tripolyphosphate (STPP), is an inorganic compound with the chemical formula Na5P3O10, is an amorphous water-soluble linear polyphosphate that is commonly used in food products as a moisture retention agent, quality improver, pH adjuster, and metal chelator.

General description
| Chemical formula | Na5P3O10 | Structure | 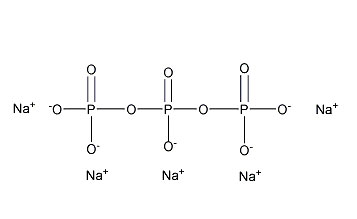 | Molecular mass | 367.864 |
| CAS | 7758-29-4 | EINECS | 231-838-7 | Melting point | 622 ℃ |
| Water solubility | Soluble in water. 20 g/100 mL (20ºC) | Appearance | White crystalline powder | Solubility | H2O: may be clear to slightly hazy |
| PH | 9.0-10.0 (25℃, 1% in H2O) | Storage | Storage temperature: no restrictions. | hs code | 2835311000 |
| Sensitiveness | Hygroscopic | Density | 2.52 g/cm³ | Odors | Odorless |
| Physical and chemical properties | Easily soluble in water, its aqueous solution is alkaline. | Stability | Stable. Incompatible with strong oxidizing agents, strong acids. Hygroscopic. | inchi | InChI=1S/H5O10P3/c1-11(2,3)9-13(7,8)10-12(4,5)6/h(H,7,8)(H2,1,2,3)(H2,4,5,6)/p-5 |
| Merck | 14,8697 | MDL | MFCD00003514 | Safety Description | S24/25;S26;S36 |
| Hazard symbols | Hazard description | R36/37/38 |
Categorization
1,Classification according to purity
Food grade sodium tripolyphosphate:
The purity of this kind of sodium tripolyphosphate is between 95% and 99%, in line with the food safety standards, commonly used in the food industry as additives, such as water retention agent, quality improvement agent, pH adjusting agent, and metal chelating agent.

Please note: Proper use of sodium tripolyphosphate is safe, but excessive intake or exposure can be harmful. To know the details please check the artical is sodium tripolyphosphate safe .
Industrial grade sodium tripolyphosphate:
The purity of industrial grade sodium tripolyphosphate is between 90% and 95%. It is suitable for non-food industry, such as detergents, water treatment agents, leather pre-tanning agents, dyeing auxiliaries and so on.
2, According to the crystallization form
Type I (high temperature type):
- Density 2.62g/cm³
- This kind of sodium tripolyphosphate is formed at high temperature with higher thermal stability and moisture absorption. Its dissolution speed is fast, but it has a large thermal effect when hydrated to form hexahydrate, and it is easy to absorb moisture and agglomerate in the atmosphere.
Type II (low temperature type):
- Density 2.57g/cm³
- Compared with type I, type II sodium tripolyphosphate is formed at lower temperatures, and its thermal stability and hygroscopicity are relatively low.
3, According to the existence of the form
Anhydrous (Na₅P₃O₁₀):
This form of sodium tripolyphosphate does not contain water of crystallization, usually is white powder.
Hexahydrate (Na₅P₃O₁₀-6H₂O):
This form of sodium tripolyphosphate contains six molecules of water of crystallization, usually presenting a right-angled parallel hexahedral crystal.
4, Other classification
According to the different production processes and uses, sodium tripolyphosphate can be further subdivided into other types, such as sodium tripolyphosphate used in detergents may pay more attention to its dispersing, emulsifying, chelating and other properties; while sodium tripolyphosphate used in the food industry needs to be strictly controlled in terms of its purity and safety.

Routine Testing Indicators
In the testing of sodium tripolyphosphate, routine test indicators are key to ensuring product quality and safety.
The following is a detailed description of these indicators:
- Whiteness: Whiteness is a measure of the color of the appearance of sodium tripolyphosphate, usually expressed in terms of reflectance or transmittance.
- Phosphorus pentoxide (P2O5): Phosphorus pentoxide is another form of phosphorus expressed in sodium tripolyphosphate.
- Sodium tripolyphosphate (STPP): This is the main component of the product, and the determination of its content is directly related to the purity and quality of the product.
- Water-insoluble substance: Water-insoluble substance refers to the part of sodium tripolyphosphate that is insoluble in water under certain conditions.
- Iron content: Iron is a common impurity element that may be introduced in the production process.
- PH: PH is a measure of the acidity or alkalinity of a solution and is particularly important for alkaline substances such as sodium tripolyphosphate.
- Granularity: Granularity refers to the size distribution of sodium tripolyphosphate particles.
These routine testing indicators form the basis for quality control of sodium tripolyphosphate, ensuring the performance and safety of the product in various applications.
Testing Methods
There are various testing methods for sodium tripolyphosphate, each with its own characteristics and applicable scenarios:
- Titration:This is a traditional chemical analytical method for determining the content of sodium tripolyphosphate by acid-base titration.
- Spectroscopic methods:These include ultraviolet-visible spectrometry (UV-VIS), atomic absorption spectrometry (AAS) and inductively coupled plasma mass spectrometry (ICP-MS).
- Chromatography: The methods include high performance liquid chromatography (HPLC) and ion chromatography (IC).
- Mass spectrometry (MS): It provides highly sensitive and selective analysis, suitable for the detection of complex samples, especially in the field of trace analysis.
Properties of sodium tripolyphosphate
1, Sodium tripolyphosphate decomposes in water to form disodium hydrogen phosphate and sodium dihydrogen phosphate.
Na5P3O10 + 2H2O → 2Na2HPO4 + NaH2PO4
It can coordinate with calcium, magnesium, iron, and other metal ions to form soluble complexes.
2, Sodium tripolyphosphate turns into polyphosphate in acidic environments, which creates a large burden and promotes calcium loss. This also indicates that sodium tripolyphosphate should not be used in acidic foods.
Applications
Sodium tripolyphosphate uses are very wide, including chelating, suspending, dispersing, gelling, emulsifying, pH buffering, etc. It can be used as the main auxiliaries of synthetic detergents, industrial water softeners, leather pre-tanning agents, dyeing auxiliaries, dispersants in the pharmaceutical industry and food additives.

History
The history of sodium tripolyphosphate dates back to the late 1800s, when people discovered the detergent properties of phosphates and began using them instead of soap.
The first industrial production of sodium tripolyphosphate
In 1907. Fritz Ullmann invented a method to prepare sodium tripolyphosphate from phosphate.
Sodium Tripolyphosphate Raw Material Sources
The preparation of sodium tripolyphosphate requires the use of some specific raw materials, mainly including the following:
1, Phosphate Ore
Phosphate ore is one of the important raw materials for sodium tripolyphosphate. Phosphate deposits are the main source of phosphate ores. The common minerals include apatite and phosphorite. Through processing, the intermediate product of phosphoric acid is extracted from the ore.

2, Phosphoric acid
Phosphoric acid is one of the raw materials for sodium tripolyphosphate. Phosphoric acid can be obtained by reacting phosphate ore or phosphate with acid. Phosphoric acid is used as an intermediate in the synthesis of sodium tripolyphosphate.
3, Sodium hydroxide
Sodium hydroxide is also one of the raw materials of sodium tripolyphosphate. It is a kind of alkaline substance, which can react with phosphoric acid to produce sodium tripolyphosphate. Sodium hydroxide can also be used as a neutralizer of phosphoric acid to assist in the formation of phosphoric acid intermediates.
Industrial Preparation Methods of Sodium Tripolyphosphate
The industrial preparation methods of sodium tripolyphosphate mainly include two main methods: thermal phosphoric acid and wet phosphoric acid.
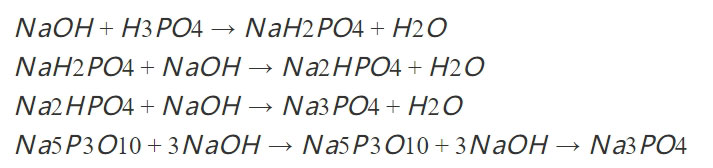
- Thermal phosphoric acid is produced by oxidizing and hydrating yellow phosphorus. Thermal phosphoric acid one-step method has the highest purity.
- Wet phosphoric acid is produced by the reaction of phosphorus ore powder and sulfuric acid extraction.
There are strict standards for the manufacturing of this chemical. Especially for the food grade sodium tripolyphosphate, and these supplied by Whwanze goes even further that has better indexes than standard required.

