Yes, sodium monofluorophosphate can whiten the teeth.
To explain the reason, we need to know what stains the teeth.
Plaque and dental calculus are the two major causes leading the teeth staining.
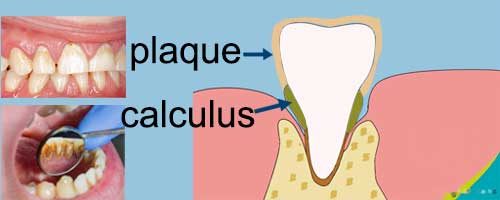
Plaque:
Plaque is a biofilm that adheres to the surface of the teeth. It consists of bacteria and substrate, and is the causative factor of caries and periodontal disease.
Looking with the naked eye, it shows no fixed shape, its texture is soft. It is a kind of sticky dirt-like object, accumulating on the surface of the teeth, it looks like a thick layer of soft dirt.
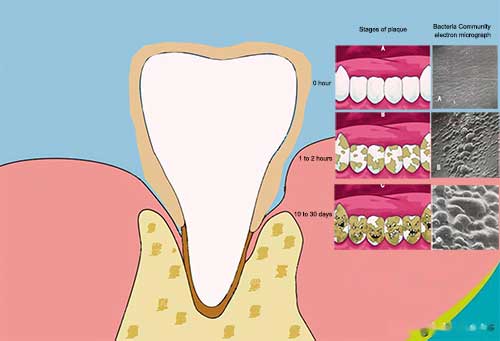
In the early stages of formation, it is usually colorless and transparent. After about 1 to 2 hours, the bacteria community will grow big. It becomes yellow after it has been on the tooth for more than 9 days. Then after 10 to 30 days, the plaque will form into dental calculus.
Plaque is so tightly bound to the surface of the teeth that it cannot be removed by rinsing, but can only be eliminated mechanically, such as by brushing, flossing, and scaling.
The main component of tooth enamel is hydroxyapatite (HAP), and the acids produced by plaque combine with hydroxyapatite(OH⁻), and eventually calcify into calculus. On the one hand, it damages the enamel, and on the other hand, it also forms stones that are more difficult to remove.
Since plaque is formed from the nutrients of saliva that bacteria gather on the surface of the teeth, and since we have to eat every day, new plaque is formed every day.
Therefore, eliminating plaque is not a one-time solution, but is a long-term process, which is why we need to insist on brushing our teeth every day.
Dental calculus (Tartar)
Tartar is the product of plaque calculus, which is yellow or dark brown. It is more difficult to eliminate than plaque and can no longer be effectively removed by brushing with regular toothpaste.
Plaque and tartar are also major culprits of bad breath.

Other causes:
There are other reasons causing toothstain, such as drug-induced tooth discoloration. But they are only take a small part of the population.
Sodium monoflurophosphate can remove the plaque and reduce the calculus !
Sodium Monofluorophosphate toothpaste not only cleans away plaque but also calculus, which is why in terms of whitening effect, sodium monofluorophosphate toothpaste is better than other toothpastes.
SMFP a type of monofluorophosphate, it contains the phosphate ion (PO43-) and Fluoride ion (F–) will give good performance on tooth whitening.
Phosphate ion (PO43-)
Inhibit growth of plaque and calculus
The PO43- can combine with the calcium ion (Ca2+) in the plaque and dental calculus, which can effectively prevent them from growing, thus achieving the effect of teeth whitening.
To prolong the generating time of the calculus could reduce the difficulty of removing the plaque and calculus.
Dissolve the plaque and calculus in the dead corner area
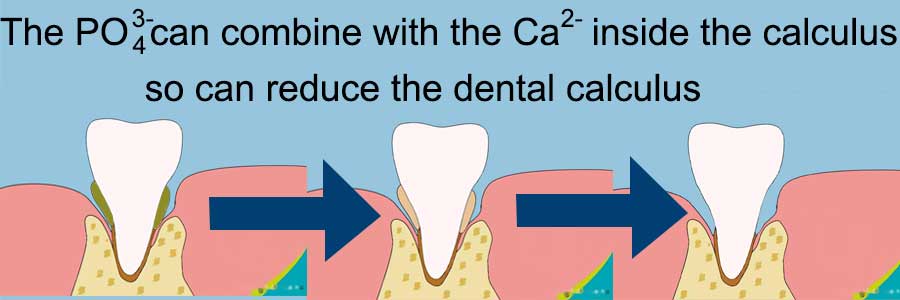
These plaque hiding in the dead corner can not be directly contacted by the toothbrush, but the phosphate component in sodium monofluorophosphate can be combined with calcium ions in the plaque, which can effectively reduce the generation of plaque.
The phosphate ion (PO43-) can combine with the calcium ions in the dental calculus, which can effectively reduce the generation of dental calculus, thus achieving the effect of teeth whitening.
The principle of binding of monofluorophosphate to calcium ions mainly involves interactions between the ions and the formation of chemical bonds.
Fluoride ions (F–)
The principle of binding of monofluorophosphate to calcium ions mainly involves interactions between the ions and the formation of chemical bonds.
In this process, the fluoride ion (F-) replaces the hydroxyl group (OH-) in hydroxyapatite to form a more stable fluorapatite.
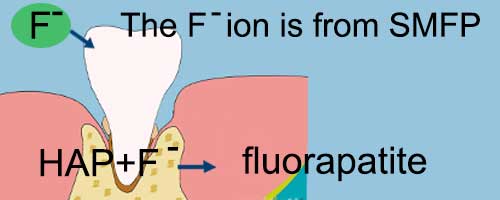
Fluorapatite has a much lower solubility and thus prevents the loss of enamel components due to acids produced by plaque. This will enhance to proof effect against the plaque and calculus forming.
In the toothpaste industry, toothpaste grade sodium monofluorophosphate is typically used in calcium-containing toothpastes, where the caries-preventive effect is assessed by testing the effective fluorine content. The principle of this combination is based on the instability and slow release of fluoride ions and the stability of fluorine in fluorapatite, which is more stable than hydroxyapatite.
Beside the whitening effect, the sodium monofluorophosphate’s mainly effect, such as to prevent the tooth from decaying and so on.
Conclusion
Compared to regular toothpastes, these containing sodium monofluorophosphate can slowly remove existing calculus and also slow down the occurrence of calculus. Therefore, it is correct to say that sodium monofluorophosphate toothpaste can whiten teeth. Furthermore, the sodium monofluorophosphate function in toothpaste not only includes whitening teeth, but also in decay proof, which is the main purpose of using this chemical. The toothpaste grade sodium monofluorophosphate supplied by Whwanze has been proofed to be a high quality raw material for the big brands toothpaste. And you’re welcome to contact us and send RFQ on this chemical.
The post is the partial response to the followings:
- sodium monofluorophosphate goodness.
- sodium monofluorophosphate positive effects.
- What does sodium monofluorophosphate help with.
- sodium monofluorophosphate benefits.
- Is sodium monofluorophosphate good for your teeth?