I. Introduction
a.Molecula formula
Sodium acid pyrophosphate is also known as di-sodium dihydrogen pyrophosphate anhydrous and its abbreviation is SAPP. It CAS No.is 7758-16-9 and EC NO. is 231-835-0.
The chemical formula of sodium acid pyrophosphate is Na2H2P2O7, which shows that the molecular structure contains phosphate (P2O7) and sodium (Na+) ions, as well as two hydrogen (H+) ions. This gives it a molecular weight of 221.95.
b.Chemical formula structure
The chemical structure is shown in the figure.
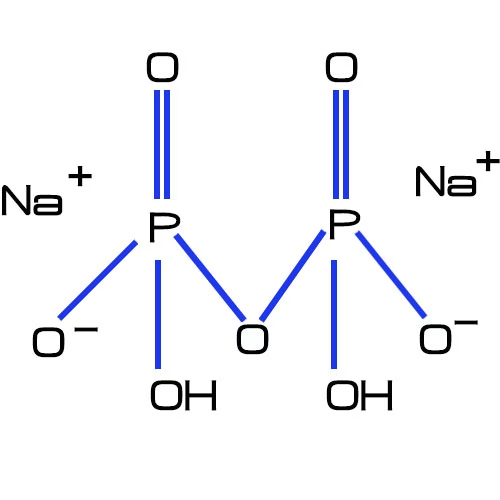
c. Producing formula
Sodium acid pyrophosphate is generally made by neutralization and polymerization reaction, taking the process and reaction formula of food grade sodium acid pyrophosphate as an example:
Drying and polymerization two-step method:
Processes:
- Add food-grade soda ash to the neutralizer, stirr and heat to dissolve it.
- Add food-grade phosphoric acid for the neutralization, control the reaction endpoint Ph = 4 ~ 4.4, resulting in sodium dihydrogen phosphate.
- Filter sodium dihydrogen phosphate solution at 70 ~ 80 ℃.
- Evaporate and concentrate the filtrate.
- Cool ,crystallize, centrifuge, dry and dehydrate at 95 ℃ to get anhydrous sodium dihydrogen phosphate.
- Send to the box-type polymerization furnace heating melt polymerization, control the material temperature at 140 ~ 200 ℃ for polymerization.
- Crush and pack the finished food grade sodium acid pyrophosphate product.
Chemical reaction formulas
H3PO4 + Na2CO3 → Na2HPO4 + H2O + CO2
2Na2HPO4 → Na4P2O7 + H2O

II. Chemical formula effects
a. Effect on properties
- Solubility: The solubility of acid sodium pyrophosphate in water is 5.9% at 20℃, and 32.5% when the temperature reaches 100℃. Aqueous solution is weakly acidic (pH 4.1-4.3, 1% solution). Insoluble in ethanol and other organic solvents.
- Stability: Acid sodium pyrophosphate is slightly hygroscopic, and six crystalline hydrates are formed after water absorption. When heated in acidic medium, acid sodium pyrophosphate will be hydrolyzed to orthophosphate. When heated above 220℃, it will decompose into sodium metaphosphate. Its aqueous solution is stable below 70°C, and will hydrolyze to disodium hydrogen phosphate when boiled. The aqueous solution is hydrolyzed to phosphoric acid when heated with dilute inorganic acid.
- Reactivity: In aqueous solution, it can make complexes with many kinds of metal ions. Acid sodium pyrophosphate reacts easily with other substances, especially with water and alkaline substances.
b. Effect on the use
- In the food industry, because of its hygroscopicity, it can be used for aspect poo, narrowing the rehydration time of the finished product, is the noodle gluten, with good taste. Since sodium pyrophosphate is acidic and its solubility varies greatly at different temperatures, it can be used as a double acid provider, reacting with baking soda to generate carbon dioxide gas, and then formulated with corn starch to make baking powder. For details, see “Sodium acid phosphate baking powder”.
- In the industrial fields, because of its phosphate ions can be complexed with metal ions, so it can be used for metal surface treatment, to prevent glass crystallization, to improve the toughness and softness of textiles, to improve the effect of electroplating, as well as in the water treatment of heavy metal ions complexed in the sewage. Its acidic character is then used to improve permeability in oil wells and facilitate oil extraction.
c. Effect on storage.

Due to its hygroscopicity and acidic characteristics, SAPP should be stored in a dry and ventilated warehouse, preventing moisture and heat, and stored separately from alkaline and harmful toxic substances.
Packing: Lined with polyethylene plastic bag, coated with composite plastic woven bag. It should be loaded and unloaded lightly to prevent the package from being damaged.
III. Conclusion
This is the summary of the chemical formula of acidic sodium pyrophosphate and its producing reaction. Due to its unique chemical structure, it has significantly different solubility at different temperatures. Its hydrolytic properties affect its stability. In addition it has the characteristics of phosphate complexing metal ions and has a certain acidity. This gives it a wide range of uses in the food industry and industry, especially in metal processing, glass manufacturing, oilfield development, textiles and electroplating. For storage and transportation, because of its hygroscopicity and acidity, it should be prevented from moisture, heat, and long-term exposure to air, and it needs to be sealed and packaged, and to avoid package breakage during transportation.
Related articles:

